Definition of corrosion
Metal materials under chemical or electrochemical influence of the environment are subject to destruction, which is called corrosion.
Corrosion of metals is caused by redox reactions, as a result of which metals become oxidized and lose their properties, which renders metal materials unusable.
There are 3 signs that characterize corrosion:
- Corrosion is, from a chemical point of view, a redox process.
- Corrosion is a spontaneous process that occurs due to the instability of the thermodynamic system metal - environmental components.
- Corrosion is a process that develops mainly on the surface of the metal. However, it is possible that corrosion can penetrate deep into the metal.
NATIONAL ECONOMIC IMPORTANCE OF COMBATING CORROSION
Losses from corrosion can be divided into direct and indirect. Direct losses are the cost of replaced products, the cost of protective measures and irretrievable loss of metal due to corrosion. According to experts, on a global scale these currently account for about 10...15% of steel production. Indirect – product losses as a result of leaks, decreased unit performance, contamination of the target product with corrosion products, etc.
A significant part of the power of ferrous metallurgy enterprises is spent on replenishing metal losses due to corrosion. However, this does not fully reflect the actual damage associated with the failure of metal products. Significant losses are caused by equipment failures, downtime, losses and waste in metalworking, violations of product quality and, ultimately, an increase in its cost and a decrease in labor productivity. Therefore, saving metal, improving the quality of raw materials and metal products, reducing corrosion losses is an indispensable condition for increasing production efficiency and product quality, which must be ensured on a national scale.
Types of metal corrosion
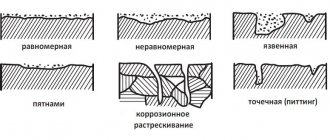
The most common types of metal corrosion :
- Uniform – covers the entire surface evenly
- Uneven
- Electoral
- Local stains – individual areas of the surface are corroded
- Ulcerative (or pitting)
- Spot
- Intercrystalline - spreads along the boundaries of a metal crystal
- Cracking
- Subsurface
Main types of metal corrosion
From the point of view of the mechanism of the corrosion process, two main types of corrosion can be distinguished: chemical and electrochemical.
How do automakers combat this?
Automotive companies have laboratories that study the processes of corrosion of body parts and offer new ways to combat corrosion. Scientists have advanced this so much that many automakers now offer a guarantee against perforation corrosion of 10 to 12 years.
But engineers still haven’t come up with anything better than galvanizing the body. And it’s unlikely that they would have come up with anything, because this is a simple and inexpensive process that allows you to cover large areas in a short time.
Electrochemical corrosion of metals
Electrochemical corrosion of metals is the process of destruction of metals in the environment of various electrolytes, which is accompanied by the appearance of an electric current inside the system.
With this type of corrosion, an atom is removed from the crystal lattice as a result of two coupled processes :
- Anodic - metal in the form of ions goes into solution.
- Cathode – the electrons formed during the anodic process are bound by a depolarizer (the substance is an oxidizing agent).
The process of removing electrons from the cathode sites is called depolarization , and substances that promote removal are called depolarizers.
The most common corrosion of metals is with hydrogen and oxygen depolarization .
Hydrogen depolarization
Hydrogen depolarization is carried out at the cathode during electrochemical corrosion in an acidic environment :
2H++2e— = H2 discharge of hydrogen ions
2H3O++2e— = H2 + 2H2O
Oxygen depolarization
Oxygen depolarization is carried out at the cathode during electrochemical corrosion in a neutral environment :
O2 + 4H++4e— = H2O reduction of dissolved oxygen
O2 + 2H2O + 4e— = 4OH—
All metals, in their relation to electrochemical corrosion, can be divided into 4 groups, which are determined by the values of their standard electrode potentials:
- Active metals (high thermodynamic instability) are all metals in the range of alkali metals - cadmium (E0 = -0.4 V). Their corrosion is possible even in neutral aqueous environments in which there is no oxygen or other oxidizing agents.
- Metals of medium activity (thermodynamic instability) are located between cadmium and hydrogen (E0 = 0.0 V). In neutral environments, in the absence of oxygen, they do not corrode, but are subject to corrosion in acidic environments.
- Low-active metals (intermediate thermodynamic stability) - are located between hydrogen and rhodium (E0 = +0.8 V). They are resistant to corrosion in neutral and acidic environments in which there is no oxygen or other oxidizing agents.
- Noble metals (high thermodynamic stability) - gold, platinum, iridium, palladium. They can be subject to corrosion only in acidic environments in the presence of strong oxidizing agents.
Types of electrochemical corrosion
Electrochemical corrosion can occur in various environments. Depending on the nature of the environment, the following types of electrochemical corrosion are distinguished:
- Corrosion in electrolyte solutions - in solutions of acids, bases, salts, in natural water.
- Atmospheric corrosion - in atmospheric conditions and in any humid gas environment. This is the most common type of corrosion.
For example, when iron interacts with environmental components, some of its sections serve as the anode, where iron oxidation occurs, and others serve as the cathode, where oxygen reduction occurs:
A: Fe – 2e— = Fe2+
K: O2 + 4H+ + 4e— = 2H2O
The cathode is the surface where the oxygen flow is greater.
- Soil corrosion - depending on the composition of the soil, as well as its aeration, corrosion can occur more or less intensely. Acidic soils are the most aggressive, while sandy soils are the least.
- Aeration corrosion occurs when there is uneven access of air to different parts of the material.
- Marine corrosion - occurs in sea water due to the presence of dissolved salts, gases and organic substances in it .
- Biocorrosion - occurs as a result of the activity of bacteria and other organisms that produce gases such as CO2, H2S, etc., which contribute to metal corrosion.
- Electrocorrosion - occurs under the influence of stray currents in underground structures, as a result of the work of electric railways, tram lines and other units.
Protective paints for metal
Applying special protective paints to a metal surface is one of the most effective means against corrosion. When dry, they form a hard film containing pigments. The thickness of this film may vary depending on the purpose of the metal product. The thickness and nature of the interaction of the paint with the surface determine the protective properties of the coating.
Anti-corrosion agents for metal can be divided into three groups:
- primers;
- paints;
- products for applying directly over rust.
When choosing a protective paint, it is important to consider the properties of the metal surface on which it will be applied. For example, for ferrous metals such as steel, it is better to choose a primer that contains zinc. The fact is that a galvanized surface can withstand destruction for a long time. As a rule, the instructions contain information about what type of surface the product is intended for.
Rust paint becomes a good solution in a situation where the surface cannot be properly cleaned of rust. It is simple and easy to use, lays down in an even dense layer. The coating created by this paint is durable and resistant to corrosion. Despite the fact that there are already pockets of corrosion on the metal surface, rust paint will not allow them to grow and spread.
Most products are suitable for manual application at home. Some paints apply better if you spray them. The composition of the paints takes into account the fact that they will be used, among other things, to protect structures located on the street. The products can be applied outdoors. As a rule, anti-corrosion paints are applied in a fairly thick layer for best effect.
The painted surface looks aesthetically pleasing. At the same time, it is reliably protected from corrosion. The film formed as a result of dyeing prevents the negative effects of light, moisture, and impurities in the atmosphere. Surface protection against oxidation is provided for up to 8 years.
Methods of protection against metal corrosion
The main method of protecting metal from corrosion is the creation of protective coatings - metallic, non-metallic or chemical.
Metal coatings
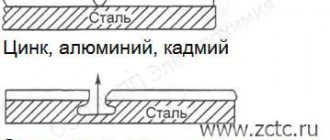
A metal coating is applied to the metal that needs to be protected from corrosion with a layer of another metal that is resistant to corrosion under the same conditions. If the metal coating is made of a metal with a more negative potential (more active) than the one being protected, then it is called an anodic coating . If the metal coating is made of a metal with a more positive potential (less active) than the one being protected, then it is called a cathodic coating .
For example, when applying a layer of zinc to iron, if the integrity of the coating is compromised, the zinc acts as an anode and will be destroyed, while the iron is protected until all the zinc is used up. The zinc coating in this case is anodic .
The cathode coating to protect the iron may, for example, be copper or nickel. If the integrity of such a coating is violated, the protected metal is destroyed.
Non-metallic coatings
Such coatings can be inorganic (cement mortar, glassy mass) and organic (high molecular weight compounds, varnishes, paints, bitumen).
Chemical coatings
In this case, the protected metal is subjected to chemical treatment in order to form a corrosion-resistant film of its compound on the surface. These include:
oxidation – production of stable oxide films (Al2O3, ZnO, etc.);
phosphating – obtaining a protective film of phosphates (Fe3(PO4)2, Mn3(PO4)2);
nitriding – the surface of the metal (steel) is saturated with nitrogen;
steel bluing - the metal surface interacts with organic substances;
carburization – obtaining on the surface of a metal its connection with carbon.
Changes in the composition of technical metal and corrosive environment
Changing the composition of the technical metal also helps to increase the metal's resistance to corrosion. In this case, compounds are introduced into the metal that increase its corrosion resistance.
Changing the composition of the corrosive environment (introducing corrosion inhibitors or removing impurities from the environment) is also a means of protecting the metal from corrosion.
Electrochemical protection
Electrochemical protection is based on connecting the protected structure to the cathode of an external direct current source, as a result of which it becomes the cathode. The anode is scrap metal, which, when destroyed, protects the structure from corrosion.
Protective protection - one of the types of electrochemical protection - is as follows.
Plates of a more active metal, called protector . The protector - a metal with a more negative potential - is the anode, and the protected structure is the cathode. The connection of the protector and the protected structure with a current conductor leads to the destruction of the protector.
Examples of problems with solutions for determining the protective properties of oxide films, determining the corrosion resistance of metals, as well as equations for reactions occurring during electrochemical corrosion of metals are given in the section Problems for the section Corrosion of metals
Categories Corrosion of metals, GENERAL CHEMISTRY
Prevention of corrosion of metal products by hot-dip galvanizing
Hot galvanizing can be called the simplest, but at the same time the oldest method of protecting metal from building structures, angles and wires.
- Surface preparation of metal products
Before applying molten zinc to the surface of steel parts, it is necessary to carry out some preparatory work. The degree of adhesion of the coating to the metal depends on how high-quality the preparation of the products is. Preparatory work is carried out in several stages. First, the metal surface is degreased, then washed, etched and fluxed.
Thorough degreasing of the surfaces of metal products is necessary to remove oil stains and various contaminants. Acids and alkaline solutions are often used for this operation. Depending on the nature of the contamination, certain reagents can be used for degreasing. This operation is carried out at temperatures between 60 °C and 80 °C. After this, the metal product must be thoroughly rinsed to remove any remaining reagents, foam, grease, etc.
At the next stage, the part is etched. To remove rust and scale residues, the product is immersed in an HCl solution with a concentration of 120 - 210 g/l. This operation ensures effective cleaning of metal parts, which is a necessary condition for ensuring adhesion of the zinc coating to the metal. At the same time, to prevent the acid from destroying the workpiece, special inhibitors are included in its solution to prevent the absorption of hydrogen (hydrogenation).
After etching is complete, the part must be rinsed again to remove any remaining solution. For more economical water consumption and ease of washing, sequential baths are used.
Upon contact with water, oxides form on the surface of metal products, which are removed by fluxing. This operation allows you to completely clean the metal and obtain a passive film layer on its surface that protects against oxidation and ensures good adhesion to zinc.
For fluxing, a composition including NH4Cl and ZnCl2 is used. For example, fluxes containing 55.4% ammonium chloride, 6% glycerin and 38.4% zinc chloride are often used in production. This procedure is performed with a concentrated solution (400 - 600 g/l) at 60 °C. In this case, it is necessary to constantly monitor the composition of the solution and promptly clean the bath (to do this, add H2O2 to it). When hydrogen peroxide is added, Fe3+ salts settle in the bath, which are collected in special settling tanks and filtered.
- Drying metal products before hot galvanizing
After fluxing, the part must be thoroughly dried. Otherwise, the remaining water on the surface of the metal, when immersed in molten zinc, begins to evaporate, which leads to micro-explosions and violates the integrity of the zinc layer. In addition, drying parts will reduce the consumption of thermal energy to maintain a stable temperature of molten zinc. The duration of the drying process exceeds the duration of galvanizing itself. To dry, metal products are placed in a drying oven heated to 100°C.
- Hot galvanizing
To ensure reliable protection of metal parts from corrosion, it is necessary to ensure the following components: high quality zinc and metal, accurate temperature control, correct preliminary preparation of the product, the required immersion/lifting speed and a certain duration of immersion, compliance with the requirements for cooling conditions.
When immersed in molten zinc, the flux melts, providing the necessary surface wettability. Dipping too slowly results in the flux melting too early and oxides appearing on the surface of the metal product. Due to too rapid immersion, the flux will not have time to melt, and the zinc coating will have defects. Therefore, it is important to strictly comply with the requirements for the speed of immersion of the product in the bath.
Hot-dip galvanizing technology involves keeping metal products in a bath of molten zinc for 3 to 10 minutes. During this time, a layer of slag forms on top of the melt. Before removing the part, it is necessary to clean it of this slag using a special scraper. Otherwise, it will settle on the galvanized product.
The speed at which a metal part is removed from molten zinc affects the thickness of the protective layer (a thicker coating is obtained with slow extraction). This is related to the rate of crystallization. The duration of lifting and tilting of the product is determined individually, based on its shape and size. The drying process of a part after hot-dip galvanizing is carried out in the open air.
To remove and further utilize HCl vapors and other harmful substances, high-power ventilation systems are installed above all baths of the production line.
The latest lines that perform the hot-dip galvanizing process operate fully automatically. Older equipment is controlled by operators using control panels, which prevents workers from coming into direct contact with harmful fumes.
Advantages of protecting metal products from corrosion using hot-dip galvanizing technology:
- a coating characterized by high corrosion resistance is formed on the surface of the part;
- affordable price;
- simplicity of the technological process;
- ease of equipment maintenance;
- high performance;
- the coating formed after hot-dip galvanizing provides protection of metal products even from mechanical damage;
- the resulting coating is characterized by high electrical and thermal conductivity;
- The hot-dip galvanizing coating prevents the metal from becoming brittle due to exposure to atomic hydrogen.
Disadvantages of technology:
- the size of parts that can be processed using the hot-dip galvanizing method is limited by the dimensions of the bath;
- galvanized metal products are poorly susceptible to further processing and welding;
- relative unevenness of the zinc layer;
- It is impossible to obtain very thin coatings using hot-dip galvanizing;
- relatively high consumption of zinc.
This technology allows you to create a protective layer on the surface of metal products from several microns to one millimeter.
SNiP norms and rules
Protecting metal structures from destruction at enterprises is a technological process in which it is necessary to comply with established standards. The official document that regulates the rules and regulations for anti-corrosion work is SNiP 2.03.11-85.
This document specifies acceptable methods for treating metal surfaces to prevent corrosion. These include:
- coating with paints and varnishes;
- impregnation with anti-corrosion compound;
- pasting with special protective films.
When performing protective work, the document prescribes taking into account the characteristics of the environment: the degree of aggressiveness, physical condition and nature of the action. For different environments, the use of materials is provided that can provide effective protection against destruction.
If the treatment of metal structures against destruction is carried out independently, the recommendations and rules from SNiP must be taken into account.
At the Cherepovets Metal Structures Plant, all work to prevent corrosion, from appropriate design to post-installation maintenance, is carried out taking into account state standards and rules. Confidence in the high quality of metal structures allows us to give our customers a guarantee of up to 24 months on all products.