A shiny pink metal with high ductility – that’s what copper is. The mineral is characterized by high electrical and thermal conductivity, lends itself well to mechanical processing and forms many compounds with other metals, which are quite widely in demand in human economic activity. In addition, copper is highly resistant to corrosion.
- Pyrometallurgical method
Its density is 8890 kg/m3.
The melting point is 10830C.
Varieties of copper ores
There are nine geological types of copper ores of industrial importance:
- Iron-nickel ores occurring in igneous rocks.
- Cuprous sandstones and shales. Stratiform reserves account for 30% of copper reserves and therefore occupy second place in this list.
- Copper-nickel. The deposits are distinguished by a variety of shapes with large inclusions of the desired metal.
- Porphyry copper. They are the undisputed leader and provide 40% of global copper production.
- Carbonatite. They are unique in that there is only one deposit in the world; in addition, they contain alkaline compounds.
- Quartz-sulfide. They do not play a significant role in ensuring production.
- Native. They are located in oxidation areas of copper-sulfide ore mines.
- Skarn. They are located among limestones and are characterized by extreme heterogeneity of morphological structure.
Copper in the listed list of ores can be presented in sulfide, oxide or mixed form, which determines the corresponding types of deposits. Based on their structure in rocks, deposits are divided into disseminated, massive and continuous textures. In the near future, this list may be supplemented by ores lying at the bottom of seas and oceans, as well as nodules of uranium deposits.
Fire refining technology for blister copper
This method of obtaining pure copper is used when the starting material is copper scrap.
The process takes place in special reverberatory furnaces, which are fired by coal or oil. The melted mass fills the bath, into which air is blown through iron pipes:
- pipe diameter – up to 19 mm;
- air pressure – up to 2.5 atm;
- oven capacity – up to 250 kg.
During the refining process, copper raw materials are oxidized, sulfur burns out, then metals. Oxides do not dissolve in liquid copper, but float to the surface. To remove them, quartz is used, which is placed in the bath before the refining process begins and is placed along the walls.
If the scrap metal contains nickel, arsenic or antimony, the technology becomes more complicated. The percentage of nickel in refined copper can only be reduced to 0.35%. But if other components are present (arsenic and antimony), then nickel “mica” is formed, which dissolves in copper and cannot be removed.
Source
Natural minerals containing copper
There are 250 copper-containing minerals in nature, but no more than 20 are used in practice. A list of the most common of them, indicating the percentage of copper:
- Native copper – 88-100%.
- Cuprite – 88.8%.
- Tenorit – 79.9%.
- Khalzokin – 79.8%.
- Covelline – 66.5%.
- Bornite – 52-65%
- Atakamit – 59.5%.
- Malachite – 57.4%.
- Brochantite – 56.2%.
- Azurite – 55.3%.
- Fahl ores – 22-53%.
- Enargit – 48.3%.
- Chrysocolla – 32.8-40.3%.
- Chalcopyrite – 34.5%.
- Cubanite – 22-24%.
Educational materials
It is used in its technically pure form in the electrical industry, and non-electrorefined copper is used for the production of alloys.
- sulfide ores, rocks copper pyrite or chalcopyrite: 60-90% pyrite FeS2 and 1-3% copper in the form of Cu2S, CuS compounds. Up to 80% of copper is obtained from sulfide ores. Cu2O – cuprite.
- oxidized ores, from which up to 15% Cu is extracted (CuO, Cu2O – cuprite; CuСO3 Cu(ОН)2 – azurite).
- native copper occurs in approximately 5%.
90% of Cu is recovered by pyrometallurgical methods. About 10% of copper is recovered by leaching low-grade ores - a hydrometallurgical method.
Copper ore mining
Copper is one of the very first metals mastered by mankind. At the very beginning, it was mined by collecting nuggets, and then they learned to extract it from ores. Over the years, mining technologies have improved. But the determining factor when choosing a mining method has always been and is the depth of the deposits. However, there are specially developed standards that take into account many factors and allow you to choose the most successful solution from an economic point of view, in terms of choosing the working depth of development and the technologies used.
In career
If the seam of the mineral being developed is located at a depth of no more than 500 m, the most appropriate is the open-pit mining method. It is with its help that most copper ores are extracted. Despite a number of problems associated with the development of a large area, the movement of huge masses of waste rock, the use of a significant amount of technical equipment and the harmful impact on the environment, the method is characterized by fairly high efficiency and the absence of significant losses of minerals. The ratio of metal yield to mined ore is: 1:200.
After conducting preliminary geological studies at the site of the future quarry or open-pit mine, the upper layers of rock are removed and disposed of in dumps. Very often this is accompanied by drilling of solid rock masses and blasting operations. The fossil mineral is extracted in layers with the further development of new massifs. Ore is picked up by bucket equipment (excavators, loaders) and loaded onto vehicles (conveyors, dump trucks) for transportation to processing plants.
In the mines
If the desired ore is located at a depth of about 1 km, then a closed mining method is used, that is, the construction of a mine and the organization of vertical, inclined or horizontal workings. Using mining equipment and drilling equipment, copper-bearing layers are developed. After which the extracted rock is loaded and removed to the surface. For this purpose, underground structures are equipped with elevators, lifting equipment, and railway tracks.
Copper
The method is quite expensive, but at the same time it provides access to deep-seated deposits.
Drilling of the wells
There is a third method for extracting copper ores - by pumping leaching solutions of acids and alkalis deep into a pre-drilled well. The result is a semi-liquid mixture, extracted to the surface by powerful pumps and subjected to further processing.
Materials scientist
To obtain copper, copper ores are used (copper content - 1...6%), as well as waste of copper and its alloys.
Copper in nature is found in the form of sulfur compounds (CuS, Cu2S), oxides (CuO, Cu2O), hydrocarbonates (Cu(OH)2), carbon dioxide compounds (CuCO3) in sulfide ores and native copper metal.
The most common ores are copper pyrite and copper luster, containing 1...2% copper.
90% of primary copper is obtained by pyrometallurgical method, 10% by hydrometallurgical method.
The hydrometallurgical method is the production of copper by leaching it with a weak solution of sulfuric acid and subsequent separation of copper metal from the solution. The method is used when processing low-grade ores; it does not allow the extraction of precious metals along with copper.
The production of copper by the pyrometallurgical method consists of beneficiation, roasting, smelting for matte, purging in a converter, and refining.
Enrichment of copper ores is carried out by flotation and oxidative roasting.
The flotation method is based on the use of different wettability of copper-containing particles and waste rock. The essence of flotation is the selective adhesion of certain mineral particles suspended in an aqueous medium to the surface of air bubbles, with the help of which these mineral particles rise to the surface. The method allows you to obtain copper powder concentrate containing 10...35% copper.
Copper ores and concentrates containing large amounts of sulfur are subjected to oxidative roasting. In the process of heating the concentrate or ore to 700...800 0C in the presence of atmospheric oxygen, sulfides are oxidized and the sulfur content is reduced by almost half the original value. Only poor concentrates (with a copper content of 8...25%) are fired, and rich (25...35% copper) concentrates are melted without firing.
After roasting, the ore and copper concentrate are smelted into matte, which is an alloy containing copper and iron sulfides (Cu2S, FeS). The matte contains 20...50% copper, 20...40% iron, 22...25% sulfur, about 8% oxygen and admixtures of nickel, zinc, lead, gold, and silver. Depending on the chemical composition of the ore and its physical state, matte is produced either in shaft furnaces, if the raw material is lump copper ore containing a lot of sulfur, or in reverberatory furnaces, if the starting product is powdered flotation concentrate. Most often, smelting is carried out in fiery reverberatory furnaces. The temperature in the smelting zone is 1450 0C.
The resulting copper matte, in order to oxidize sulfides and iron, is subjected to blowing with compressed air in horizontal converters with side blast. The resulting oxides are converted into slag, and sulfur into SO2. Heat in the converter is released due to chemical reactions without fuel supply. The temperature in the converter is 1200…1300 ºC. Thus, the converter produces blister copper containing 98.4...99.4% copper, 0.01...0.04% iron, 0.02...0.1% sulfur and a small amount of nickel, tin, antimony, silver, gold. This copper is poured into a ladle and poured into steel molds or a casting machine.
Blister copper is refined to remove harmful impurities, followed by fire and then electrolytic refining.
The essence of fire refining of blister copper is the oxidation of impurities that have a greater affinity for oxygen than copper, removing them with gases and converting them into slag. After fire refining, copper with a purity of 99...99.5% is obtained. It is poured into molds and ingots are obtained for further smelting of alloys (bronze and brass) or ingots for electrolytic refining.
Electrolytic refining is carried out to obtain copper free of impurities (99.95% Cu).
Electrolysis is carried out in baths where the anode is made of fire-refined copper, and the cathode is made of thin sheets of pure copper. The electrolyte is an aqueous solution of CuSO4 (10...16%) and H2SO4 (10...16%).
When a direct current is passed, the anode dissolves, the copper goes into solution, and copper ions are discharged at the cathodes, depositing a layer of pure copper on them.
Impurities are deposited at the bottom of the bath in the form of sludge, which is processed to extract metals: silver, antimony, selenium, tellurium, gold, etc...
The cathodes are unloaded after 5...12 days, when their mass reaches 60...90 kg. They are thoroughly washed and then melted in electric furnaces.
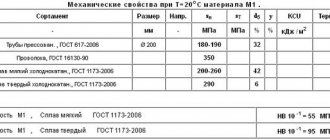
Copper by purity is divided into grades: M0 (99.95% Cu), M1 (99.9%), M2 (99.7%), M3 (99.5%), M4 (99%).
Copper production
After mining the ore, the next problem arises: how to extract the necessary material from it? There are several ways.
One of the oldest technologies involved burning malachite ores with limited air access. The mass placed in pots, mixed with coal, burned, releasing carbon monoxide. Which led to achieving the desired result - obtaining copper that was sufficiently pure for its time.
It is clear that over the past centuries, methods and methods of ore processing have undergone major changes, driven by the goal of achieving the most optimal results for any type of primary raw material. That is why modern metallurgy is based on three main methods for producing copper.
Pyrometallurgical method
Based on high-temperature processes, the pyrometallurgical method is ideally suited for sulfide ores, which are sometimes quite poor in copper concentration. It allows you to extract metal even with a content of 0.5%.
But first of all, the feedstock is enriched during the flotation process. Its essence lies in thoroughly grinding the ore, filling it with water, and adding complex organic flotation reagents. They envelop mineral particles containing copper alloys, giving them non-wetting.
At the second stage of this process, foam is created in the solution, the bubbles of which pick up particles coated with organic matter. This happens under the influence of air flow, as a result of which the formations float to the surface, from where they are later taken up. The foam saturated with copper compounds is collected, squeezed out and dried.
After which the resulting concentrate is fired at a temperature of 14,000 C. This is necessary to remove sulfur and oxidize sulfides. Then high-temperature (14,0000 - 15,0000C) smelting is carried out in shaft furnaces to produce an alloy of iron and copper - matte. Next, in the process of Bessemer smelting in a converter under the influence of oxygen, the oxide is obtained, and then the blister copper itself, containing 90.95% of the metal. In this case, sulfur turns into an acid residue, and iron into silicate slag.
You can obtain pure copper from a rough substance using:
- fire refining,
- electrolysis,
- exothermic reduction reaction under the influence of hydrogen.
Hydrometallurgical method
To extract copper and a number of other metals from polymetallic ores containing less than 0.5% of the desired mineral, the hydrometallurgical method is used.
The extracted minerals are dissolved using non-concentrated sulfuric acid or ammonia. Copper is obtained from the resulting liquids during the displacement reaction. Metallic iron is used to carry out the reaction.
Electrolysis method
The method is designed to obtain pure copper through an electrolytic reaction.
Its technology is to produce pure copper thin sheet cathodes and thick plate anodes from blister copper. Placed then in a bath filled with copper sulfate, they react under the influence of electric current. Copper dissolves on the anodes and is deposited on the cathodes. Released impurities are removed by chemical methods.
Copper pipes
Stages of pyrometallurgical copper production
This method is effectively used for processing ore with different copper contents. It consists of the following sequence of actions:
- preparation (enrichment) of extracted raw materials;
- direct smelting onto matte;
- converting the resulting matte;
- final refining.
Each technological process is carried out using the necessary processing methods. To isolate blister copper, so-called purging is performed. Next, the copper is placed into molds or poured onto slabs. It remains contaminated with various impurities and does not have the properties of pure copper.
The essence of the process is to supply air under pressure through the liquid melt of copper matte. It is produced in special converters, which can be positioned vertically or horizontally. Subsequently, enriched copper ore concentrates are sent for final processing.
Enrichment
Initially, the copper content in the mined ore does not exceed six percent. To produce copper with the best efficiency, it is necessary to enrich the mined ore. This production is intended to obtain a concentrate that will contain more than 10% copper. In some cases it can be increased to 35%.
The main method of beneficiation of sulfide copper-nickel ores is flotation. To increase the efficiency of enrichment, a magnetic separation operation is first performed. It promotes the release of pyrrhotite into an independent concentrate. The possibility of carrying out magnetic separation is due to the relatively high magnetic susceptibility of pyrrhotite.
Areas of use
There are many industries where this oldest of metals finds its application:
- Metallurgy. It is this industry that produces many finished products in the form
- rolled products: sheets, plates, strips, pipes, rods, tires, wire;
- alloys: bronze, brass, cupronickel, constantan, nickel silver manganin.
Both products and intermediate materials are widely used in technical industries, in the production of weapons, and in the arts and crafts. The distinctive features of the alloys are the preservation of mechanical properties, a high level of slip in a paired combination and anti-corrosion resistance.
- Mechanical engineering. A significant portion of copper-containing products obtained as a result of metallurgical processes is used here. These are high-strength alloys with aluminum, tin, silicon, and zinc. As well as various parts of machines and mechanisms. One of the directions is the production of hard solders, which again find application in the mechanical engineering industry.
- Chemistry. The catalyst for the polymerization of acetylene is again copper.
- Electrical engineering. Due to its high electrical conductivity, this metal has become indispensable as a conductor in the manufacture of buses, cables, wires, and printed circuit board tracks. They, in turn, are part of many electrical products, which also contain copper structural elements and alloys of this metal. In addition, copper is used in chemical current sources and in the manufacture of high-temperature superconducting materials.
- Energy. One of the important areas of using copper is the production of pipes based on it, which are an integral part of gas supply systems, water supply, heating, cooling, air conditioning and supply of process fluids.
- Jewelry making. The specifics of manufacturing precious items that serve as jewelry require a combination of a number of contradictory factors. To give strength to gold, copper is added to it. The pliability of the material does not decrease, but the service life and resistance to mechanical stress increase significantly.
Technology
Bessemerizing is an iron smelting process that produces steel of relatively high quality. It should be noted that such technology is used extremely rarely today. This is due to the emergence of quite a large number of modern technologies that make it possible to obtain higher quality steel in less time.
The entire Bessemer steel production process can be divided into several main stages:
- Cast iron is poured into the converter through the neck. An important point is that in this position the device must be in a horizontal position, since there is a possibility that the nozzle will be filled with metal. Nozzles are necessary to blow through the charge. It is the oxidation of impurities and their removal as slag that makes it possible to obtain steel of improved quality.
- The next stage is to start the blast and turn the converter into a vertical position.
- In order to ensure the oxidation of harmful impurities and excess carbon, the metal is blown with air. At this stage, slag is formed, with which unnecessary chemicals are removed.
- After a sufficiently long period of purging, the converter is turned over again into a horizontal position, and the purging of molten metal stops.
- The molten metal is drained into a ladle and deoxidized by adding special substances.
At the time of the start of purging the composition, active oxidation of manganese and silicon occurs. At the initial stage, carbon is practically not oxidized. This is due to the fact that this component reacts exclusively to exposure to high temperatures. In addition, the process of oxidation of impurities is influenced by thermodynamic factors that determine the activity of oxygen transfer to the sites of the Bessemer process.
Considering this technology, we note the following points:
- At the first stage, a large number of different slags are formed, which contains a high concentration of silica. The time interval for the first stage is 2-5 minutes.
- The second stage of the Bessemer production process provides the most favorable conditions for carbon oxidation. An example is an increase in operating temperature to approximately 2000 degrees Celsius. The duration of this stage is approximately 13 minutes. At the end of this stage, the temperature drops to approximately 1600 degrees Celsius.
- High quality steel can be achieved using various bessemerization methods. It all depends on the characteristics of the composition of the scrap used and the concentration of the cream in the composition.
- In order to eliminate the possibility of metal overblowing, the active air supply is stopped already at the second stage.
- Only at the third stage can active oxidation of iron be noted, which causes the release of brown smoke. This stage begins at the moment when the carbon concentration is less than 0.1%.
As previously noted, the Bessemer method of steel production has become widespread due to its high productivity. Foundries quite often install equipment that has a charge of about 35 tons.
Bessemer method of steel smelting
Today, the Bessemer method of steel production is practically not used, which is due to the low quality of the resulting metal and its fairly high cost.
Source
Deposits in Russia and the world
On the territory of Russia there are many fairly large deposits of copper ore:
- Allarechenskoye, Monchegorskoye, Pechenga - Murmansk region.
- Gayskoye - Orenburg region.
- Mikheevskoye, Tominskoye – Chelyabinsk region
- Yubileiny, Sibayskoye, Podolskoye, Zapadno-Ozernoye, Uchalinskoye, Novo-Uchalinskoye, Oktyabrskoye - Republic of Bashkortostan.
- Bystrinskoye and Udokanskoye - Transbaikalia.
- Oktyabrskoye, Talnakhskoye - Krasnoyarsk Territory.
The following deposits of this mineral are highlighted on the world map:
- Chuquicamata, Escondida, Collahhuasi, Antamina, El Tesoro - Chile.
- Bingham Canyon, Keweenaw, Pebble - USA.
- Vale Salobu - Brazil.
- Nurkazgan - Kazakhstan.
- Uyu Tolgoi - Mongolia.
- Grazberg - Indonesia.
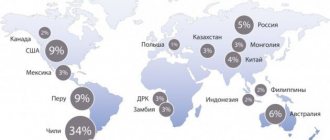
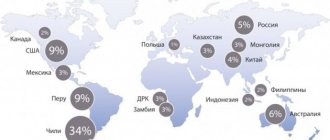
Copper mining countries
The leading positions in global copper production (2022 data in quantitative terms of the metal mined per year) are occupied by:
- Chile – 5.8 million tons.
- Peru – 2.4 million tons.
- China – 1.6 million tons.
- USA – 1.2 million tons.
- Congo - 1.2 million tons.
Judging by expert estimates, the total volume of as yet unexplored copper reserves in the world is 3.5 billion tons. These reserves should be enough for the next century and a half.
Author: Yuri Florinskikh All articles by this author
Latest articles by the author: The largest producers of milk and dairy products in the world Diamonds: properties, mining methods and applications
Features of copper: its composition, structure and production technology
Copper, which belongs to non-ferrous metals, has been known for a long time. Its production was invented before people began to make iron.
According to assumptions, its active use occurred as a result of its availability and fairly simple extraction from copper-containing compounds and alloys.
So, let's look today at the properties and composition of copper, the world's leading countries in copper production, the manufacture of products from it and the features of these areas.
Copper has a high coefficient of electrical conductivity, which has increased its value as an electrical material. If previously up to half of all copper produced in the world was spent on electrical wires, now aluminum is used for these purposes as a more affordable metal. And copper itself becomes the most scarce non-ferrous metal.
This video discusses the chemical composition of copper:
The structural composition of copper includes many crystals: nickel, gold, calcium, silver, lead and many others. All metals included in its structure are distinguished by relative softness, ductility and ease of processing. Most of these crystals, when combined with copper, form solid solutions with continuous rows.
The unit cell of this metal is cubic in shape. For each such cell there are four atoms located at the vertices and the central part of the face.
Chemical composition
The composition of copper during its production may include a number of impurities that affect the structure and characteristics of the final product. At the same time, their content should be regulated both by individual elements and by their total quantity. Impurities that are found in copper include:
- Bismuth. This component negatively affects both the technological and mechanical properties of the metal. That is why it should not exceed 0.001% of the finished composition.
- Oxygen. It is considered the most undesirable impurity in copper. Its maximum content in the alloy is up to 0.008% and rapidly decreases when exposed to high temperatures. Oxygen negatively affects the ductility of the metal, as well as its resistance to corrosion.
- Manganese. In the case of the manufacture of conductive copper, this component negatively affects its conductivity. Already at room temperature it quickly dissolves in copper.
- Arsenic. This component creates a solid solution with copper and has virtually no effect on its properties. Its action is largely aimed at neutralizing the negative effects of antimony, bismuth and oxygen.
- Nickel. Forms a solid solution with copper and at the same time reduces its thermal and electrical conductivity.
- Tin. Creates a solid solution and enhances thermal conductivity.
- Selenium, sulfur. These two components have the same effect on the final product. They form a fragile connection with copper and amount to no more than 0.001%. As the concentration increases, the degree of ductility of copper sharply decreases.
- Antimony. This component is highly soluble in copper and therefore has minimal impact on its final properties. It is allowed no more than 0.05% of the total volume.
- Phosphorus. Serves as the main deoxidizer of copper, the maximum solubility of which is 1.7% at a temperature of 714°C. Phosphorus, in combination with copper, not only promotes better welding, but also improves its mechanical properties.
- Zinc. Contained in a small amount of copper, it has virtually no effect on its thermal and electrical conductivity.
The process and proper sequence of copper production will be discussed next.