About the properties of iron
Pure iron is silver-gray in color and has ductility and malleability. Native ingots found in nature have a pronounced metallic luster and significant hardness. The electrical conductivity of the material is high; it easily transmits current with the help of free electrons. The metal has average refractoriness, softens at a temperature of +1539 degrees Celsius and loses its ferromagnetic properties. This is a chemically active element. At normal temperatures it easily reacts, and when heated, these properties increase. In air it becomes covered with a film of oxide, which interferes with the continuation of the reaction. When exposed to a humid environment, rust appears, which no longer prevents corrosion. But despite this, iron and its alloys are widely used.
Brief characteristics of iron and nickel
Iron in its pure form has a silver-gray color, is ductile, malleable. Its nuggets have a noticeable metallic luster and are also distinguished by significant hardness. Electrical conductivity is also at a high level, because the metal easily transmits current due to free electrons.
Iron has medium refractoriness and becomes soft at a temperature of +1,539 °C, which is why it loses its ferromagnetic properties. This element is chemically active, so under normal conditions it quickly reacts with other substances.
As the temperature rises, these properties become even more pronounced. When exposed to air, it oxidizes, as a result of which an oxide film appears on its surface - it is this film that stops further reaction. With increased humidity, rust forms on iron, which does not prevent the metal and alloys based on it from being actively used in industry.
VT-metall offers services:
Nickel nuggets are found in iron meteorites, and in more common conditions this metal is found in combination with other chemical elements. More precisely, to obtain an alloy consisting of iron and nickel, the latter component is obtained from sulfide, copper-nickel ores:
- nickel – in addition to nickel, contains arsenic;
- chloanthite is a white pyrite containing cobalt and iron;
- garnierite – silicate rock with a share of magnesium;
- magnetic pyrite – is a mixture of sulfur, iron, copper;
- gersdorffite – has an arsenic-nickel luster;
- pentlandite – contains sulfur, iron, nickel.
The choice of method for obtaining nickel from ore depends on the type of raw material. It is worth noting that in some cases the metal of interest to us acts as a secondary material for enriching the rock.
A little history
Invar is an alloy of iron and nickel, which contains 36% alloying additive. It was first discovered in France in 1896 by physicist Charles Guillaume. At this time, he was working on the search for inexpensive metal for standards of mass and length measures, which were made from a very expensive platinum-iridium alloy. Thanks to this discovery, the scientist received the Nobel Prize in physics in 1920.
The word "invar" in Latin means unchanging. This means that the coefficient of thermal expansion of an iron-nickel alloy remains constant over a wide temperature range - from -80 to 100 degrees Celsius. This alloy has several other names: nilvar, vakodyl, nilo-alloy, radiometal. Invar is a trademark of Imphy Alloys Inc., which is owned by the steel company Arcelor Mittal.
Nickel grades and chemical composition
According to GOST 849-2008, 7 grades of nickel are produced - N0, N1Ау, Н1у, Н1, Н2, Н3 and Н4. They contain from 97.6 to 99.99% nickel in total with a small percentage of cobalt (Co) - from 0.005 to 0.7%. The rest of the mass is occupied by impurities:
- Carbon (C) - found in all grades of nickel.
- Magnesium (Mg).
- Aluminum (Al).
- Silicon (Si).
- Phosphorus (P).
- Sulfur (S) - found in all brands.
- Manganese (Mn).
- Iron (Fe).
- Copper (Cu) - found in all brands.
- Zinc (Zn).
- Arsenic (As)
- Cadmium Cd).
- Tin (Sn).
- Antimony (Sb).
- Lead (Pb).
- Bismuth (Bi).
The detailed chemical composition of different grades of nickel is presented in the table below.
| Brand | Chemical composition, % | |||||||||||||||||
| Ni and co, no less | Including Co, no more | Impurities, no more | ||||||||||||||||
| C | Mg | Al | Si | P | S | Mn | Fe | Cu | Zn | As | Cd | Sn | Sb | Pb | Bi | |||
| H0 | 99,99 | 0,005 | 0,005 | 0,001 | 0,001 | 0,001 | 0,001 | 0,001 | 0,001 | 0,002 | 0,001 | 0,0005 | 0,0005 | 0,0003 | 0,0003 | 0,0003 | 0,0003 | 0,0001 |
| H1Ay | 99,95 | 0,1 | 0,001 | 0,001 | — | 0,002 | 0,001 | 0,001 | — | 0,01 | 0,1 | 0,001 | 0,001 | 0,0006 | 0,0005 | 0,0005 | 0,0005 | 0,0001 |
| H1y | 99,95 | 0,1 | 0,01 | 0,001 | — | 0,002 | 0,001 | 0,001 | — | 0,01 | 0,015 | 0,001 | 0,001 | 0,0005 | 0,0005 | 0,0005 | 0,0005 | 0,0003 |
| H1 | 99,93 | 0,1 | 0,01 | 0,001 | — | 0,002 | 0,001 | 0,001 | — | 0,02 | 0,02 | 0,001 | 0,001 | 0,001 | 0,001 | 0,0001 | 0,001 | 0,0006 |
| H2 | 99,8 | 0,15 | 0,02 | — | — | 0,002 | — | 0,003 | — | 0,04 | 0,04 | 0,005 | — | — | — | 0,1 | — | |
| H3 | 98,6 | 0,7 | 0,1 | — | — | — | — | 0,03 | — | — | 0,6 | — | — | — | — | — | — | |
| H4 | 97,6 | 0,7 | 0,15 | — | — | — | — | 0,04 | — | — | 1,0 | — | — | — | — | — | — | |
Iron-nickel alloy
To improve the properties of iron, alloys are prepared using various additives. Scientists believed that it would not be difficult to obtain an iron-nickel alloy, taking into account the thermodynamic properties of metals. But in practice they encountered problems. When metals interact during the production of an alloy of iron and nickel, as a result of a side oxidation process, iron passes from the divalent state to the trivalent state.
As a result, the yield of the alloy decreases and certain physical properties deteriorate. To solve this problem, amines and organic acids are added to the electrolyte, which form compounds with low solubility with ferric iron. In this regard, the elasticity of the sediment becomes better, and the electrolytes are mixed to distribute it evenly. The resulting alloy of iron and nickel is called Invar.
Structure of the resulting alloy
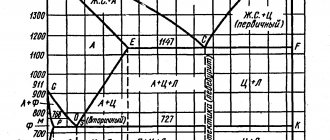
When melted, the iron-nickel alloy is dissolved solid iron in a nickel base. Due to this connection, it is possible to increase the temperature of structural stability by 200 °C. Penetration of nickel into iron occurs when it reaches +500 °C, and acceleration is observed only at +800 °C.
The FeNi3 component is the main structural element of the alloy, so the nickel content reaches up to 55%. This effect is associated with the processing temperature of the material. It is worth mentioning that the maximum share of nickel is 60%.
It is important that iron alone in the alloy does not give the material the necessary properties. The important temporary stability of invar is explained by the presence of carbon and other impurities. These include cobalt, chromium, carbon, manganese, phosphorus. Also, the alloy of iron and nickel may contain silicon, sulfur, aluminum, magnesium, zirconium, and titanium. Their share is largely determined by alloy specification and production. Thus, superinvar includes approximately 5% cobalt, which is achieved by eliminating 5% nickel. Another version of the alloy - kovar - contains 54% iron, 29% nickel, 17% cobalt.
Application of Invar alloy
Its low temperature coefficient of expansion allows it to be used for the production of:
- parts of control and measuring instruments;
- tapes and wires for geodetic work;
- laser supporting structures;
- parts of clock mechanisms, chronometer pendulums;
- rolled products: hot-rolled rod and sheet, cold-rolled strip, seamless pipes, forged rods.
To increase strength, cold plastic deformation of an iron-nickel alloy is performed, and then low-temperature heat treatment is performed. For greater resistance to corrosion under normal atmospheric conditions, its surface is polished and a protective layer is applied if the product is intended for use in aggressive environments. The anti-corrosion properties of Invar will also increase by adding about 12% chromium to its composition, while it retains constant elasticity when heated to 100 degrees.
Magnetic alloys
These alloys are widely used in electrical engineering. They are used to make permanent magnets, transformer cores, electrical measuring instruments, and electromagnets. People have long known that iron has magnetic properties and as a result it has many uses.
Much later it was discovered that the same property is inherent in nickel and some other metals. Products made from a magnetic alloy of iron and nickel also have the ability to retain their own magnetic field when the external one is no longer present. Moreover, this personal field is again capable of influencing other magnetic bodies.
Nickel-based alloys
Nickel is an element of the tenth group, the fourth period of the periodic table of chemical elements of D.I. Mendeleev, with atomic number 28. It is designated by the symbol Ni (lat. Niccolum). The simple substance nickel is a ductile, malleable, transition metal of a silvery-white color; at ordinary temperatures in air it is covered with a thin film of oxide. Chemically inactive.
In modern turbines and jet engines, the most important part is the turbine blade (Fig. 43). The power of a jet engine largely depends on the maximum temperature of the working fluid (gas) at which the blades can operate for a long time. In modern jet engines, turbine blades heat up to 700 - 900 ° C, and there is a tendency to increase this temperature.
Austenitic steels and alloys based on nickel and cobalt are used for turbine blades.
Nickel alloys are predominantly used, containing, as a rule, chromium (in an amount of about 15 - 20%) and other quite numerous additives, although in much smaller quantities (aluminum, titanium, tungsten, molybdenum, vanadium, etc.).
Rice. 43 Products made of nickel alloys
Like austenitic steels, nickel-based alloys can be divided into homogeneous (called nichromes and inconels ) and aging (called nimonics ).
Nichrome is the generic name for a class of nickel-based alloys intended for resistors and heating elements. The most common brand is X 20H80.
Nichrome is an alloy of nickel and chromium, which, depending on the brand of the alloy, contains 55-78% nickel (Ni); 15-23% chromium (Cr); 1.5% manganese (Mn); iron, aluminum, etc. make up the remainder. Due to the higher nickel content, X20N80 is more expensive. Typical nichrome products are nichrome wire and nichrome tape (Fig. 44). Nichrome wire is made with a diameter of 0.3 to 10 mm. The sizes of nichrome tape range in thickness from 0.1 to 3.2 mm and width from 6 to 250 mm.
Rice. 44 Nichrome products
Nickel alloys with additives of alloying elements - Ti, Al, Cr, used for the manufacture of the most stressed parts of gas turbine engines and other power plants operating at high temperatures. Nickel at 800° has a long-term strength of 100 hours. about 4 kg/mm2, the 20% Cr additive strengthens the solid solution relatively little, but increases the scale resistance of the alloy; the increase in long-term strength does not exceed 25-30%. The introduction of Ti into nichrome alloys in an amount of 2.5–3% with a small addition of aluminum (0.7%) provides a significant increase in their heat resistance due to the formation of a highly dispersed intermetallic γʹ phase at moderate temperatures; As a result of dispersion hardening processes, the resistance of alloys to plastic deformation increases both at room and at high temperatures. A slight increase in aluminum content (in the presence of titanium addition) further increases the heat-resistant properties of nickel-based alloys due to an increase in the amount of dispersed phases formed during heat treatment.
One of the factors determining heat resistance is high creep resistance. The heat resistance of alloys is assessed by the limits of long-term strength or creep at high temperatures, and is associated, first of all, with their structure and composition. In structure, heat-resistant alloys must be multiphase with strong grain and phase boundaries. In nickel heat-resistant alloys, the above is ensured by multicomponent alloying. In this case, the higher the heat resistance of alloys, the higher the volume fraction of the strengthening phases and the higher their thermal stability, that is, resistance to dissolution and coagulation with increasing temperature.
High resistance to corrosion in a number of aggressive environments, heat resistance, high ohmic resistance and some other special properties of nickel and its alloys determine the use of these materials in chemical engineering, electrochemical and other industries. In the manufacture of welded equipment for the chemical industry, mainly H0 grade nickel is used (at least 99.93% Ni). On average, for this nickel in the annealed state, the tensile strength is 38–45 kgf/mm2, the relative elongation is 32–50%. Alloys of nickel with copper, chromium, aluminum and other elements are used in welded products.
Welding of nickel alloys
Welding of nickel and its alloys is made difficult primarily by the tendency for grain growth at high temperatures. At the same time, low-melting brittle eutectics are located along the boundaries of large grains: Ni3S-Ni with a melting point of 645°C and Ni3P-Ni with a melting point of 880°C. The appearance of these eutectics reduces the strength of grain boundaries and during crystallization, in the semi-liquid state of the metal, hot cracks appear under the action of tensile stresses.
The second serious problem is the formation of gas pores. Hydrogen and, to a lesser extent, nitrogen dissolve well in nickel. In addition, hydrogen is able to interact with nickel oxide NiO and form water. Nitrogen reacts with nickel oxide NiO and forms volatile nitrides Ni3N. Carbon also reacts with NiO to form CO. These processes lead to the formation of pores.
The third problem when welding nickel and its alloys is the high surface tension of molten nickel, which reduces the depth of metal penetration. This requires increasing the cutting angle of the edges of the parts or using activating fluxes.
For welding under a layer of flux (Fig. 45), wires of the NMts2.5, NP-0, NP-1, Np-2 grades and oxygen-free salt fluxes of type 48-OF-6 and ANF-573 are used.
Rice. 45 Submerged arc welding of nickel-based alloys
Corrosion resistance of nickel alloys
Nickel is characterized by a favorable combination of properties: high corrosion resistance in many aggressive environments, high mechanical properties, good workability in hot and cold conditions. Nickel has the ability to dissolve many elements in large quantities, such as chromium, molybdenum, iron, and copper. The most important alloying elements in corrosion-resistant nickel alloys are chromium, molybdenum and copper.
The corrosion resistance of some nickel alloys is associated with passivity, while others are due to the fact that they have a fairly high equilibrium potential and do not replace hydrogen in acidic environments. This explains the large number of environments in which nickel alloys can be successfully used: acids, salts and alkalis (both oxidizing and non-oxidizing in nature), sea and fresh water, as well as the atmosphere .
Corrosion-resistant nickel alloys belong to the following three main alloying systems: Ni-Mo; Ni-Cr and Ni-Cr-Mo .
Modern high-alloy, weldable, structurally stable, corrosion-resistant nickel-based alloys include:
- - nickel-molybdenum alloys [grades N65M-VI (EP982-VI), N70MFV-VI (EP814A-VI), Hastelloy B-2, Nimofer S6928], having exceptionally high resistance in non-oxidizing environments - in hydrochloric, phosphoric, sulfuric acids, wet hydrogen chloride, organic acids at elevated temperatures;
- — nickel-chromium-molybdenum alloys [grades KhN63MB (EP758U), KhN65MVU (EP760), KhN56MD, Hastelloy C-276, Hastelloy C-22, Nicrofer 5923hMo], which have high corrosion resistance in a wide range of highly aggressive environments of an oxidizing and reducing nature; in aqueous solutions of copper chlorides (up to 20%) and iron (up to 35%); solutions of sulfuric, phosphoric, acetic and formic acids contaminated with chlorine and fluorine ions; in dry chlorine; wet hydrochloride gas; in silicon hydrofluoric acid; in mixtures of acids and other aggressive environments;
- - nickel-chromium alloys [grades KhN58V (EP795), Nicrofer6030], having high resistance in solutions of nitric acid in the presence of fluorine at high temperatures.
The alloy compositions are characterized by a balanced content of basic (Mo, Cr, Cr + Mo) and additional (V, W, Fe) alloying elements, and are also regulated by a low content of impurity elements (C, Si, S, P). This provides the alloys with high resistance against general corrosion in appropriate environments and against various types of local corrosion, and manufacturability in the manufacture of various types of metallurgical and engineering products.
Additional material for the nickel section, nickel-based alloys of their grades and recommendations for use
Modern various industrial grades of nickel.
- H0, H1 - Primary nickel, Ni+Co content - not less than 99.99% and 99.93%, respectively. Nickel of these grades is produced in the form of cathode sheets, plates, and strips. These alloys are obtained using electrolysis.
- H2, H3, H4 - Primary nickel, Ni+Co content - not less than 99.8%, 98.6% and 97.6%, respectively. Nickel of these grades is produced in the form of plates, strips, cathode sheets, granules, edges and ingots. These alloys are produced using electrolysis, remelting, pressing of nickel waste, and fire refining.
- NP1, NP2, NP3, NP4 - semi-finished nickel, Ni+Co content - not less than 99.99%, 99.5%, 99.3% and 99.0%, respectively. Nickel of these grades is produced in the form of nickel wire, rods, sheets, strips and tapes.
- NPA1, NPA2 - semi-finished anode nickel, Ni+Co content - not less than 99.7%, 99.0%, respectively. Nickel of these grades is produced in the form of sheets and rods.
- NPAN - semi-finished anodic non-passivating nickel (a thin film with high resistance does not form on the surface of products made of nickel of this grade), Ni+Co content - no less than 99.4%. Nickel of this grade is produced in the form of rods and sheets.
- NK0.2 - Silicon nickel, Ni+Co content - not less than 99.4%. Nickel of this grade is produced in the form of wire.
- NMC1, NMC2, NMC2.5, NMC5 - Manganese nickel, contains up to 98.5% Ni+Co (grade NMC1). Nickel of these grades is produced in the form of wire.
Grades and application of nickel-based alloys.
| No. | Brand | Application |
| 1 | ХН65МВУ (EP760) | for the manufacture of welded chemical equipment (columns, heat exchangers, reactors). |
| 2 | ХН65МВ (EP567) | for the manufacture of welded chemical equipment, operating under the most severe conditions. |
| 3 | NP2 | for the manufacture of welded chemical equipment in the production of liquid chlorine, chlorine, caustic soda, etc. |
| 4 | N70MFV-VI (EP814A-VI) | for the manufacture of welded chemical equipment (tanks, heat exchangers, reactors), operated at elevated temperatures. |
| 5 | HN55MBYU (EP666) | for the manufacture of stamped, welded and soldered products operating at temperatures from 750 °C to -253 °C. |
| 6 | ХН58В (EP795) | for the manufacture of welded equipment operating in nitric acid solutions. |
| 7 | N65M-VI (EP982-VI) | for the manufacture of welded chemical equipment, used in particularly aggressive environments of a reducing nature. |
| 8 | HN63MB (EP758U) | for the manufacture of welded chemical equipment (in the chemical, petrochemical, pulp and paper and other industries). |
| 9 | NP1A-ID | for the manufacture of welded chemical equipment in the production of liquid chlorine, chlorine, caustic soda, etc. |
| 10 | NP1A | for the manufacture of welded chemical equipment in the production of liquid chlorine, chlorine, caustic soda, etc. |
479
Nickel, cobalt and their alloys
Cobalt and nickel are elements of the iron subgroup. All three elements have similar properties, but there are also significant differences. Both metals have a higher density than iron and are much harder and stronger. They are less active chemically and are corrosion resistant. In addition, metals are valued for their greater resistance to gas corrosion.
The disadvantages of cobalt and nickel are their high toxicity and significant cost relative to iron. They find their application for anti-corrosion external coating of products made of carbon steel and iron through electrochemical reactions. They are also used for the manufacture of components and parts that require enhanced strength and hardness. It should be noted the special importance of alloys of iron, nickel and cobalt, which are called coinvar, invar, supermalloy, permalloy and malloy. Their main advantage is their high magnetic properties. These alloys are used to produce magnetic cores for various electromagnetic devices.
The influence of impurities on the properties of the metal
Sulfur is one of the most harmful impurities. It gives nickel red brittleness, due to which the properties of the metal deteriorate during pressure treatment. To neutralize the effect of sulfur, manganese and/or magnesium are added.
Carbon in amounts up to 0.1% does not affect the properties of the metal in any way, however, with a higher content of this element, it falls out of the solid solution during annealing and reduces the ductility of cold nickel.
When the content of bismuth and lead is in an amount of 0.002%, hot metal processing becomes impossible: since these elements are almost insoluble in the solid state, they destroy the ingot. Therefore, in all grades of nickel, the amount of lead and bismuth is limited to 0.001 and 0.0006%, respectively.
Aluminum increases the electrical resistance of nickel. This element is contained in the purest grade - H0. In addition, nickel and aluminum alloys are widely used: they have high heat resistance and corrosion resistance.
Iron does not have a noticeable effect on the properties of nickel. Silicon deoxidizes the base metal, due to which it has a beneficial effect on its casting properties, chemical resistance and strength.
Cobalt increases the heat resistance, heat resistance and strength of nickel, and manganese has a positive effect on the technological and mechanical properties of the metal and improves its electrical resistance.
Kovar alloy
The mixture consists of metals with excellent mechanical properties. They are easy to process and can be easily rolled, drawn, forged and stamped. An alloy of cobalt, nickel and iron is otherwise called kovar. A well-chosen combination of chemical elements provides the material with excellent characteristics. This alloy has good thermal conductivity, a high coefficient of electrical resistivity and linear expansion rates close to zero over a wide temperature range. The only drawback is low corrosion resistance in damp environments, so protective coatings made of silver are often used. Kovar is widely used in industry for the production of:
- pipes, tapes and wires;
- capacitors;
- equipment housings in instrument making;
- parts in radio electronics;
- housings in the electrovacuum industry.
The content of expensive cobalt and nickel in the alloy increases the cost of the material, but good characteristics and long-term operation cover the initial investment.
Properties and characteristics of heat-resistant alloys
Let's look at them using the most common brands as an example.
Alloy EP747 (or KhN45Yu) is used in metallurgy for the manufacture of roller tables along which ingots move. In addition to iron and nickel (nickel content 44...46%), it also contains chromium and aluminum . The alloy is smelted in electric furnaces, after which it undergoes hot plastic deformation, the temperature range of which is in the range of 1280...8500C (the first temperature is the beginning of deformation, the second is the end). The alloy lends itself well to heat treatment and electric arc welding. Assortment – sheets up to 2 mm thick and rods.
The physical and mechanical properties of the KhN45Yu alloy are:
- Mechanical strength – from 600 MPa at room temperatures, to 150 MPa at a temperature of 8000C;
- Heat resistance in still air – up to 1300...13500C;
- Oxidation intensity, g/m2∙h – no more than 170;
- Thermal conductivity coefficient at operating temperatures, W/m2 ∙K – 17.5…24.5;
- Young's modulus at operating temperatures, GPa – 12.5...17.5.
Alloy EI602 (or KhN75MBTYu) is used for internal lining of combustion chambers of metallurgical and thermal furnaces at temperatures not exceeding 900...9500C . In addition to iron and nickel , it also contains chromium, titanium, molybdenum, aluminum and niobium . Due to its more complex composition, which includes very heterogeneous chemical elements, after smelting in electric furnaces it is subjected to hot deformation in a much narrower temperature range: 1180...12800C. Unlike the previous alloy, KhN75MBTYu is more ductile, in particular, it allows deep drawing. hollow machine parts can be made from it , which will then be operated at high temperatures . Well welded by all types of electric welding .
Intensive formation of scale on the surface of this alloy begins only at temperatures from 1250...12800C. The alloy is supplied only in the form of sheets - hot or cold rolled.
The physical and mechanical properties of the KhN75MBTYu alloy are:
- Mechanical strength – from 860 MPa at room temperatures, to 177 MPa at a temperature of 9000C;
- Long-term strength and thermal endurance, MPa, not less than - 190;
- Thermal conductivity coefficient at operating temperatures, W/m2 ∙K – 20.2…19.3;
- Young's modulus at operating temperatures, GPa – 19.0...10.2.
Alloy EI868 (or KhN60VT) is distinguished by even higher heat resistance and resistance to exposure to aggressive environments . Therefore, it is used for the manufacture of gas turbine blades operating at temperatures of 950...10000C . The chemical composition of the alloy contains tungsten and chromium , and titanium .
Alloy range – sheets, rods and wires. The alloy has machinability and weldability characteristics similar to the KhN75MBTYu alloy, but is distinguished by higher heat resistance, the highest of the heat-resistant iron-nickel alloys: the oxidation intensity at operating temperatures of 10000C does not exceed 0.6...0.8 g/m2∙h. The structure and strength of the alloy do not change even after 30...35 heating and cooling cycles. The remaining physical and mechanical properties of the EI868 alloy are:
- Mechanical strength – from 800 MPa at room temperatures, to 43 MPa at a temperature of 10000C;
- Long-term strength and thermal endurance, MPa, no less - 210;
- Thermal conductivity coefficient at operating temperatures, W/m2 ∙K – 28…24;
- Young's modulus at operating temperatures, GPa – 19.0…2.0.
Alni alloys
Alni is the group name for iron-nickel-aluminum magnetic alloys. As the concentration of aluminum and nickel increases within certain limits, the residual induction decreases and the coercive force increases. The most commonly used alloys are those containing 11 to 18% aluminum and 20–34% nickel. The main properties of such alloys are electrical conductivity, thermal conductivity and ductility. All of them are characterized by good welding.
To use alloys in the manufacture of magnets, they are alloyed with cobalt and copper. In this case, the material becomes hard and brittle and has a coarse-grained structure. Alni alloys are used as a structural material for parts of gas turbine and jet engines operating under high temperatures of more than 1000 degrees Celsius for a long time, preserving the metal without damage.
Concept of precision alloys
Precision alloys differ from any other in that in them the base metal acquires pre-selected additional characteristics. They have unique physical, chemical and mechanical properties. You need to understand that the characteristics of the alloy depend on the content of each of the components in it, such as iron, nickel, copper, cobalt and other metals.
Separately, it is worth mentioning precision alloys with “anomalous properties,” as they are usually designated. Their physical characteristics can be maintained or undergo minimal changes under the influence of such external factors as:
- temperature;
- magnetic and electric fields, and this is where their properties such as amplitude, frequency, phase, polarization are important;
- change in the level of mechanical load;
- reactive environments.
The most commonly used are approximately twelve precision alloys, including: elinvar, constantan, perminvar, manganin, and invar. Invar is an alloy of nickel and iron, which is discussed in this article.