| Lead | |
| Atomic number | 82 |
| Appearance of a simple substance | |
| Properties of the atom | |
| Atomic mass (molar mass) | 207.2 a. e.m. (/mol) |
| Atomic radius | 175 |
| Ionization energy (first electron) | 715.2 (7.41) kJ/mol () |
| Electronic configuration | [Xe] 4f14 5d10 6s2 6p2 |
| Chemical properties | |
| Covalent radius | 147 |
| Ion radius | (+4e) 84 (+2e) 120 |
| Electronegativity (Pauling) | 1,8 |
| Electrode potential | Pb←Pb2+ -0.126 V Pb←Pb4+ 0.80 V |
| Oxidation states | 4, 2 |
| Thermodynamic properties of a simple substance | |
| Density | 11,3415 /³ |
| Molar heat capacity | 26.65[1]/(mol) |
| Thermal conductivity | 35,3 /(·) |
| Melting temperature | 600,65 |
| Heat of Melting | 4.77 kJ/mol |
| Boiling temperature | 2 013 |
| Heat of vaporization | 177.8 kJ/mol |
| Molar volume | 18.3 ³/mol |
| Crystal lattice of a simple substance | |
| Lattice structure | cubic face-centered |
| Lattice parameters | 4,950 |
| c/a ratio | n/a |
| Debye temperature | 88,00 |
| Pb | 82 |
| 207,2 | |
| [Xe]4f145d106s26p2 | |
| Lead | |
Lead
- an element of the main subgroup of the fourth group, the sixth period of the periodic system of chemical elements of D.I. Mendeleev, with atomic number 82. Denoted by the symbol Pb (Latin: Plumbum). The simple substance lead (CAS number: 7439-92-1) is a malleable, relatively fusible gray metal.
The origin of the word "lead" is unclear. In most Slavic languages (Bulgarian, Serbo-Croatian, Czech, Polish) lead is called tin. A word with the same meaning, but similar in pronunciation to “lead”, is found only in the languages of the Baltic group: švinas (Lithuanian), svins (Latvian). The Latin plumbum (also of unclear origin) gave the English word plumber - plumber (once pipes were caulked with soft lead), and the name of the Venetian prison with a lead roof - Piomba, from which, according to some sources, Casanova managed to escape. Known since ancient times. Products made from this metal (coins, medallions) were used in Ancient Egypt, lead water pipes - in Ancient Rome. Lead is referred to as a specific metal in the Old Testament. Lead smelting was the first metallurgical process known to man. Until 1990, large quantities of lead were used (together with antimony and tin) to cast typographical fonts, and also in the form of tetraethyl lead to increase the octane number of motor fuels.
Chemical properties of lead
Electronic formula: KLMN5s25p65d106s26p2, according to which it has oxidation states +2 and +4. Lead is not very reactive chemically. A metallic section of lead shows a metallic luster, which gradually disappears due to the formation of a thin film of PbO.
With oxygen it forms a number of compounds Pb2O, PbO, PbO2, Pb2O3, Pb3O4. Without oxygen, water at room temperature does not react with lead, but at high temperatures lead oxide and hydrogen are produced by the interaction of lead and hot water vapor.
The oxides PbO and PbO2 correspond to the amphoteric hydroxides Pb(OH)2 and Pb(OH)4.
The reaction of Mg2Pb and dilute HCl produces a small amount of PbH4. PbH4 is an odorless gaseous substance that very easily decomposes into lead and hydrogen. At high temperatures, halogens form compounds of the type PbX2 with lead (X is the corresponding halogen). All these compounds are slightly soluble in water. Halides of the PbX4 type can also be obtained. Lead does not react directly with nitrogen. Lead azide Pb(N3)2 is obtained indirectly: by reacting solutions of Pb(II) salts and NaN3 salt. Lead sulfides can be obtained by heating sulfur with lead, PbS sulfide is formed. Sulfide is also obtained by passing hydrogen sulfide into solutions of Pb(II) salts. In the voltage series, Pb is to the left of hydrogen, but lead does not displace hydrogen from dilute HCl and H2SO4, due to the overvoltage of H2 on Pb, and films of poorly soluble PbCl2 chloride and PbSO4 sulfate are formed on the metal surface, protecting the metal from further action of acids. When heated, concentrated acids such as H2SO4 and HCl act on Pb and form with it soluble complex compounds of the composition Pb(HSO4)2 and H2[PbCl4]. Nitric acid, as well as some organic acids (for example, citric acid) dissolve lead to produce Pb(II) salts. According to their solubility in water, lead salts are divided into insoluble (for example, sulfate, carbonate, chromate, phosphate, molybdate and sulfide), slightly soluble (for example, chloride and fluoride) and soluble (for example, lead acetate, nitrate and chlorate). Pb(IV) salts can be obtained by electrolysis of solutions of Pb(II) salts strongly acidified with sulfuric acid. Pb(IV) salts add negative ions to form complex anions, for example, plumbates (PbO3)2- and (PbO4)4-, chloroplumbates (PbCl6)2-, hydroxoplumbates [Pb(OH)6]2- and others. Concentrated solutions of caustic alkalis react with Pb when heated, releasing hydrogen and hydroxoplumbites of the X2[Pb(OH)4] type. Eion (Me=>Me++e)=7.42 eV.
Receipt
Basic source of Pb – sulfide polymetallic. ore. By enriching ores (1–5% Pb) by flotation, lead concentrates are obtained (containing 40–75% Pb, 5–10% Zn, up to 5% Cu, noble metals and Bi). The technology for producing sulfide includes agglomerating roasting of sulfide concentrates, and mine recovery. smelting of the agglomerate and refining of rough carbon. During firing, PbS is oxidized by oxygen from the air blown into the melt containing flux additives (SiO2, CaCO3, Fe2O3): 2PbS+3O2=2PbO+2SO2. The finished agglomerate contains 35–45% Pb and 1.2–3% S (including in the form of sulfates). The agglomerate is mixed with coke and sent for reduction. smelting in shaft furnaces into which air or an air-oxygen mixture is supplied. A two-stage exothermic process occurs in the furnace. reaction (2PbS+3O2=2PbO+2SO2 and PbS+2PbO=3Pb+SO2), the product of which is rough S. (Pb recovery reaches 90–94%). Waste from the smelting process (slag) is sent for further processing to extract Zn. The dust generated during shaft smelting (and agglomeration) serves as the raw material for the extraction of rare and trace elements. Chernova S. contains 93–98% Pb and impurities: Cu (1–5%), Sb, As, Sn (0.5–3%), Bi (0.05–0.4%), etc. Purification of rough S. produce pyrometallurgical. or electrolytic way. The volume of world production of S. is approx. 9 million tons/year.
Main lead compounds
Lead oxides
Lead oxides are predominantly basic or amphoteric in nature. Many of them are painted red, yellow, black, and brown. In the photograph at the beginning of the article, on the surface of the lead casting, tarnish colors are visible in its center - this is a thin film of lead oxides formed due to the oxidation of hot metal in air.
Lead halides
Lead chalcogenides
Lead chalcogenides—lead sulfide, lead selenide, and lead telluride—are black crystals that are narrow-gap semiconductors.
Lead salts
Lead sulfate Lead nitrate Lead acetate
- Lead sugar is a very toxic substance. Lead acetate, or lead sugar, Pb(CH3COO)2·3H2O exists in the form of colorless crystals or white powder, which slowly erodes with the loss of water of hydration. The compound is highly soluble in water. It has an astringent effect, but since it contains poisonous lead ions, it is used externally in veterinary medicine. Acetate is also used in analytical chemistry, dyeing, calico printing, as a silk filler, and for the production of other lead compounds. Basic lead acetate Pb(CH3COO)2·Pb(OH)2, a less water-soluble white powder, is used to decolorize organic solutions and purify sugar solutions before analysis.
Cosmetics, plumbing, typographical letters...
In the British Museum there is a lead figurine of a woman from Egypt, dating back to the third millennium BC. This is the oldest (officially) lead object. The Egyptians (more or less wealthy) considered it indecent to go out without applying makeup to their eyes. Therefore, the cosmetics market was well developed. Black lead sulfide or white lead carbonate was used for eyeliner. The fat base was goose fat. The raven shine of ancient Egyptian hair was often achieved by vulgar coloring with lead oxide paste.
Great Roman Empire
The eternal city left behind eternal roads (some are still quite usable), eternal Roman law (which, despite its ancient age, is still studied by lawyers), and aqueducts. They brought water from the mountains to the residents. Lead pipes were used in the extensive water supply system.
The Romans attached great importance to water supply. Sextus Julius Frontinus, who lived in the first century AD, wrote an entire book “On Aqueducts.”
Lead pipes of an ancient Roman water supply with inscriptions
Here are a couple of quotes from it:
“The pipe is made by rolling a lead plate five fingers wide into a circle.
... All curators ... served as consuls before their appointment, which indicates the high status of the water curator.”
Time forward!
With the development of printing, more and more lead was needed. It was used for the production of typographical letters. The invention of batteries and the development of the automobile industry spurred metal mining. And everyday, but necessary little things, such as pigments and lead compounds for paints, are still in demand.
Papal bull of 1637 with lead seal
That's what they called...
The origin of the name of the metal is still unclear. In the old days, tin and lead were often confused, although enlightened people (like Pliny) distinguished them: Plumbum nigrum (lead), Plumbum album (tin). For the English, a plumber is a plumber.
Informative: in Venice there was a Piombi prison; it was also called Lead. Casanova, the lover of all times and peoples, sat in it and managed to escape from it.
In many Slavic languages our hero is called tin. Although in Old Russian it is (svinets), Slovenian (svinec), in Belarusian (svinets). But in the Baltic languages the names are similar - svinas (Lithuanian), svin (Latvian).
There is a version: the word has the same root as the word “pig”, pig, pig - an ingot of dirty metal. We recommend: MANGANESE - the dream of steelworkers
Lead Applications
Lead in the national economy
Lead nitrate
used for the production of powerful mixed explosives.
Lead azide is used as the most widely used detonator (initiating explosive). Lead perchlorate is used to prepare a heavy liquid (density 2.6 g/cm³) used in flotation beneficiation of ores, and it is sometimes used in high-power mixed explosives as an oxidizing agent. Lead fluoride alone, as well as together with bismuth, copper, and silver fluoride, is used as a cathode material in chemical current sources. Lead bismuthate, lead sulfide PbS, lead iodide are used as cathode material in lithium batteries. Lead chloride PbCl2 as a cathode material in backup current sources. Lead telluride PbTe is widely used as a thermoelectric material (thermo-emf with 350 μV/K), the most widely used material in the production of thermoelectric generators and thermoelectric refrigerators. Lead dioxide PbO2 is widely used not only in lead batteries, but also on its basis many reserve chemical current sources are produced, for example, lead-chlorine cell, lead-fluoride cell, etc. Lead white
, basic carbonate Pb(OH)2•PbCO3 , a dense white powder, is obtained from lead in air under the influence of carbon dioxide and acetic acid. The use of white lead as a coloring pigment is no longer as common as it once was due to its decomposition by hydrogen sulfide H2S. Lead white is also used for the production of putty, in the technology of cement and lead carbonate paper. Lead arsenate and arsenite are used in insecticide technology to kill agricultural pests (gypsy moth and cotton boll weevil). Lead borate Pb(BO2)2·H2O, an insoluble white powder, is used to dry paintings and varnishes, and, along with other metals, as coatings on glass and porcelain. Lead chloride PbCl2, a white crystalline powder, is soluble in hot water, solutions of other chlorides and especially ammonium chloride NH4Cl. It is used to prepare ointments for treating tumors. Lead chromate PbCrO4 is known as chrome yellow dye, and is an important pigment for making paints, for dyeing porcelain and fabrics. In industry, chromate is used mainly in the production of yellow pigments. Lead nitrate Pb(NO3)2 is a white crystalline substance, highly soluble in water. This is a binder of limited use. In industry, it is used in matchmaking, textile dyeing and printing, antler dyeing and engraving. Lead sulfate Pb(SO4)2, a water-insoluble white powder, is used as a pigment in batteries, lithography, and printed fabric technology. Lead sulfide PbS, a black, water-insoluble powder, is used in firing pottery and to detect lead ions. Since lead absorbs γ radiation well, it is used for radiation protection in X-ray facilities and in nuclear reactors. In addition, lead is considered as a coolant in projects of advanced fast neutron nuclear reactors. Lead alloys are widely used. Pewter (tin-lead alloy), containing 85-90% Sn and 15-10% Pb, is moldable, inexpensive and used in the manufacture of household utensils. Solder containing 67% Pb and 33% Sn is used in electrical engineering. Alloys of lead and antimony are used in the production of bullets and typographic fonts, and alloys of lead, antimony and tin are used for figured casting and bearings. Lead-antimony alloys are commonly used for cable sheaths and electric battery plates. Lead compounds are used in the production of dyes, paints, insecticides, glass products and as an additive to gasoline in the form of tetraethyl lead (C2H5)4Pb (moderately volatile liquid, vapors in small concentrations have a sweetish fruity odor, in large concentrations they have an unpleasant odor; Tm = 130 °C, boiling point = 80 °C/13 mm Hg; density 1.650 g/cm³; nD2v = 1.5198; not soluble in water, miscible with organic solvents; highly toxic, easily penetrates skin; maximum permissible concentration = 0.005 mg/m³; LD50 = 12.7 mg/kg (rat, oral)) to increase octane number.
Lead in medicine
Economic indicators
Prices for lead in ingots (grade C1) in 2006 averaged 1.3-1.5 dollars/kg.
Countries, largest consumers of lead in 2004, in thousand tons (according to ILZSG):
| China | 1770 |
| EU | 1553 |
| USA | 1273 |
| Korea | 286 |
Mining and production
According to the US Geological Bureau, the world's metal reserves reach one and a half billion tons. The main part of them is owned by the following countries:
- China;
- USA;
- Russia;
- SOUTH AFRICA;
- Peru;
- Canada;
- Mexico.
The annual production of metal in the world is about 3 million tons. China is creating a lead shortage in this market. Its demand for the metal accounts for about 45% of global production.
Our gray hero is produced from minerals and recycled materials (tons of batteries from used cars).
3.7. Thermal conductivity
The thermal conductivity coefficient λ denotes the amount of heat transferred per unit time through a unit surface with a unit temperature gradient, i.e., with a temperature difference of one degree per unit wall length normal to the heat flow.
Thermal conductivity coefficient dimension: W/(m K).
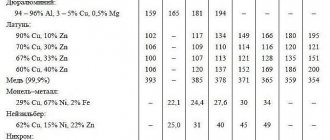
In table 3.7.1 shows the thermal conductivity coefficients of metals and alloys.
For each value of λ, the temperature to which this value corresponds is indicated. In cases where such an indication is absent, the data refers to room temperature.
Table 3.7.1
Thermal conductivity coefficients of metals and alloys
The composition of the alloys is indicated in mass fractions (except where otherwise specified).
| Metal or alloy, mass. % | T, °С | λ, W/(m K) |
| Aluminum 99 | 18 | 211 |
| 30 | 208,1 | |
| 100 | 205,2 | |
| 400 | 318,2 | |
| 600 | 422,9 | |
| Bismuth | –186 | 10,47 |
| –77 | 10,76 | |
| 0 | 7,411 | |
| 100 | 6,866 | |
| 96 Bi + 3.5 Pb (vol.) | 44 | 5,401 |
| 90 Bi + 3.5 Sn (vol.) | 44 | 5,401 |
| 80 Bi + 20 Sb | 0 | 6,364 |
| 100 | 8,583 | |
| 50 Bi + 50 Sn | 12,5 | 23,45 |
| 50 Bi + 25 Pb + 25 Sn | 20 | 16,24 |
| 48 Bi + 26 Pb + 13 Sn + 13 Cd | 7 | 13,36 |
| Tungsten | 0 | 160,4 |
| 2227 | 148,2 | |
| Iron | ||
| forged pure | 0 | 59,45 |
| 100 | 56,94 | |
| 99.92 (armco) | 20 | 73,27 |
| 100 | 67,41 | |
| Gold | 0 | 311,5 |
| 97 | 312,3 | |
| 90 Au + 10 Pd | 25 | 97,97 |
| 50 Au + 50 Pd | 25 | 36,01 |
| Iridium | 17 | 59,03 |
| Cadmium | 0 | 92,65 |
| 100 | 85,62 | |
| Potassium | 5 | 97,97 |
| 20,7 | 97,13 | |
| 57,6 | 90,85 | |
| 62.9 K + 37.1 Na | 6,0 | 22,99 |
| 42,9 | 25,92 | |
| Cobalt (97.12 Co + 0.24 C + 1.4 Fe + 1.1 Ni + 0.14 Si) | 30 | 487,8 |
| Brass | ||
| red | 0 | 103 |
| 100 | 118,5 | |
| yellow | 0 | 85,45 |
| 100 | 106,3 | |
| Lithium | 0 | 71,18 |
| 101,3 | 75,36 | |
| Magnesium | 0–100 | 157,4 |
| 92 Mg + 8 Al | 20–200 | 62,8–79,55 |
| 92 Mg + 8 Cu | 20–200 | 125,6–132,3 |
| 88 Mg + 10 Al + 2 Si | 20–200 | 121,4–133,1 |
| Manganese | 18 | 21,77 |
| Copper | –183 | 465,2 |
| 0 | 385,2 | |
| 100 | 385,2 | |
| 99.37 Cu + 0.63 P | 30 | 104,7 |
| 98.02 Cu + 1.98 P | 30 | 52,34 |
| 96 Cu + 3 Si + 1 Mn (everdure) | 20 | 33,08 |
| 84 Cu + 4 Ni + 12 Mn (manganin) | 18 | 21,73 |
| 100 | 26,42 | |
| 60 Cu + 40 Ni | 18 | 22,61 |
| 100 | 26,8 | |
| 54 Cu + 46 Ni | 18 | 20,26 |
| 89 Cu + 11 Zn | 18 | 115,1 |
| 87 Cu + 13 Zn | 18 | 126 |
| 82 Cu + 18 Zn | 18 | 131 |
| 68 Cu + 32 Zn | 18 | 108,9 |
| 62 Cu + 22 Zn + 15 Ni | 18 | |
| 52 Cu + 26 Zn + 22 Ni | 0 | 29,31 |
| 100 | 36,43 | |
| 95 Cu + 5 Al (aluminum bronze) | 20 | 82,48 |
| 90 Cu + 10 Sn | 20 | 41,87 |
| 75 Cu + 25 Sn (tin bronze) | 20 | 25,54 |
| 92.8 Cu + 5 Sn + 2 Zn + 0.15 P (phosphor bronze) | 20 | 79,13 |
| Molybdenum | 17 | 144,9 |
| Sodium | 5,7 | 134,4 |
| 21,2 | 132,7 | |
| 88,1 | 120,6 | |
| Nickel 99 | –160 | 54,01 |
| 18 | 58,62 | |
| Ni + (2÷3) Co | 300 | 52,75 |
| 79.5 Ni + 13 Cr + 6.5 Fe (niconel) | 70 | 15,07 |
| Tin | –170 | 81,64 |
| 0 | 64,06 | |
| 100 | 59,45 | |
| 91 Sn + 8.9 Zn | 44 | 65,73 |
| Palladium | 100 | 76,2 |
| 90 Pd + 10 Pt | 25 | 56,1 |
| 50 Pd + 50 Pt | 25 | 36,84 |
| 90 Pd + 10 Ag | 25 | 47,73 |
| 50 Pd + 50 Ag | 25 | 31,82 |
| Platinum | –252,8 | 389,4 |
| –183 | 76,2 | |
| 0–200 | 69,92 | |
| 90 Pt + 10 Ir | 17 | 30,98 |
| 90 Pt + 10 Rh | 17 | 30,14 |
| 90 Pt + 10 Pd | 25 | 43,12 |
| Rhodium | 17 | 87,92 |
| Mercury | ||
| hard | –269,3 | 167,5 |
| –44,2 | 27,8 | |
| liquid | 0 | 8,081 |
| 50 | 8,75 | |
| Lead | 18 | 34,62 |
| 100 | 34,12 | |
| Silver 99.9 | –160 | 417,8 |
| 0 | 458,9 | |
| 10–97 | 403,2 | |
| Silver 99.98 | 18 | 421,2 |
| 100 | 415,3 | |
| 90 Ag + 10 Pd | 25 | 141,1 |
| 90 Ag + 10 Pt | 25 | 97,97 |
| 70 Ag + 30 Pt | 25 | 30,98 |
| Steel | See table. 3.7.2 | |
| Antimony | 0 | 18,42 |
| 0–30 | 17,58 | |
| 100 | 16,75 | |
| 70 Sb + 30 Bi | 0 | 9,797 |
| 100 | 11,76 | |
| 66.7 Sb + 33.3 Cd | 0 | 1,252 |
| 50 Sb + 50 Cd | 0 | 2,173 |
| Tantalum | 17 | 54,43 |
| 1827 | 82,9 | |
| Zinc | –170 | 117,2 |
| 18 | 111 | |
| 100 | 109,7 | |
| 70 Zn + 30 Sn | 44 | 93,78 |
| Cast iron | 18 | 45,64 |
| 100 | 45,22 | |
| Metal or alloy, mass. % | T, °С | λ, W/(m K) |
In table 3.7.2–3.7.7 show the thermal conductivity coefficients of some steels, pure substances in the solid state, thermal insulation, construction and some other materials, liquid metal coolants, pure organic liquids and refrigerants in the liquid state.
Table 3.7.2
Thermal conductivity coefficients λ (W/(m K)) of some steels
| Steel group | Temperature, °C | |||||||
| 100 | 200 | 300 | 400 | 500 | 600 | 700 | 800 | |
| Carbon: grade 15 | 54,4 | 50,2 | 46,1 | 41,9 | 37,7 | 33,5 | ||
| mark 30 | 50,2 | 46,1 | 41,9 | 37,7 | 33,5 | 29,3 | ||
| Molybdenum | 41,9 | |||||||
| Chrome | 22,4 | 21,2 | 23,5 | 22 | ||||
| Chrome-molybdenum: Х10С2М (ЭИ107) | 18,4 | 0 | 21,7 | 24,7 | 22 | |||
| 12 XM | 37,7 | 35,6 | 33,5 | |||||
| Chrome-nickel | 16,9 | 19,2 | 21,5 | 24,4 | 26,7 | 29,7 | 32,6 | 36,1 |
| Chrome-nickel-tungsten | 15,5 | 0 | 18,1 | 21,2 | 22 | |||
Table 3.7.3
Thermal conductivity coefficients of some pure substances in the solid state
| Name | Formula | T, °С | λ, W/(m K) |
| Aluminum oxide: | Al2O3 | ||
| powder | 46,8 | 0,678 | |
| fused | 650–1350 | 3,349 | |
| Graphite (density 1580 kg/m3): | WITH | ||
| axes | 50 | 44,17 | |
| + axes | 142 | 17,84 | |
| 555 | 116,8 | ||
| Graphite (powder, density 700 kg/m3) | WITH | 40 | 1,193 |
| Cadmium oxide (pressed powder) | CdO | 46,5 | 0,682 |
| Potassium iodide | KI | 0 | 5,024 |
| Potassium chloride | KCl | 0 | 6,95 |
| Cobalt(III) oxide (pressed powder) | Co2O3 | 48,5 | 0,419 |
| Silicon carbide (carborundum) | SiC | 650–1350 | 15,57 |
| Silicon dioxide (quartz): | SiO2 | ||
| axes | 0 | 13,61 | |
| 100 | 9,002 | ||
| + axes | 0 | 7,247 | |
| 100 | 5,581 | ||
| Magnesium oxide (pressed powder, density 797 kg/m3) | MgO | 47,6 | 0,607 |
| Copper(II) oxide (pressed powder) | CuO | 45,6 | 1,013 |
| Sodium chloride | NaCl | 0 | 1,116 |
| Naphthalene | S10N8 | 0 | 0,377 |
| 1-Naphthol | С10Н8О | 35 | 0,293 |
| 2-Naphthol | С10Н8О | 35 | 0,335 |
| Nickel(III) oxide (pressed powder, density 1445 kg/m3) | Ni2O3 | 46,2 | 0,938 |
| Sulfur: | S | ||
| rhombic | 0 | 0,293 | |
| plastic | 20–100 | 0,264 | |
| Silver bromide | AgBr | 0 | 1,03 |
| Silver chloride | AgCl | 0 | 1,089 |
Table 3.7.4
Thermal conductivity coefficients of thermal insulation, building and some other materials
| Material | T, °С | λ, W/(m K) |
| Asbestos fabric | 20 | 0,279 |
| Asbestos fiber | 0 | 0,112 |
| 100 | 0,121 | |
| Asbestos cardboard | 100 | 0,144 |
| Asphalt | 20 | 0,744 |
| Basalt | 20 | 2,175 |
| Concrete | 20 | 0,922 |
| Bauxite | 600 | 0,557 |
| Woolen felt | 40 | 0,073 |
| Gypsum | 0 | 1,297 |
| Fireproof clay | 300–600 | 0,875–0,925 |
| Granite | 20 | 3,419 |
| Tree: | ||
| birch (10.8% humidity), + fibers | 29 | 0,172 |
| oak (density 825 kg/m3), + fibers | 15 | 0,209 |
| oak (density 819 kg/m3), ¦ fibers | 20 | 0,349 |
| Diatomaceous earth | 20 | 0,055 |
| Charcoal | 81 | 0,076 |
| Limestone | 0 | 2,07 |
| Clay lime | 20 | 3,256 |
| Coal | 20 | 0,186 |
| Corrugated cardboard | 0,064 | |
| Brick: | ||
| insulating | 100 | 0,14 |
| refractory | 200 | 1,006 |
| building | 20 | 0,233–0,291 |
| Clinker | 30 | 0,163 |
| Powdered coke | 100 | 0,191 |
| Ice | 0 | 2,25 |
| –95 | 3,954 | |
| Magnesite | 1000 | 1,663 |
| Marble: | ||
| white | 3,268 | |
| black | 30 | 2,861 |
| Boiler room scale | 65 | 1,31–3,14 |
| Onyx | 30 | 2,34 |
| Wood sawdust | 20 | 0,07 |
| Paraffin | 20 | 0,267 |
| Sand: | ||
| dry | 20 | 0,326 |
| wet | 20 | 1,13 |
| Sandstone (density 2259 kg/m3) | 40 | 1,84 |
| Portland cement | 30 | 0,302 |
| Cork granulated | 20 | 0,038 |
| Cork plate | 30 | 0,042 |
| Soft rubber | 20 | 0,167 |
| Slate | 100 | 1,49 |
| Mica | 0,582 | |
| Snow: | ||
| freshly fallen | 0,105 | |
| compacted | 0,048 | |
| Glass wool | 0 | 0,037 |
| Textolite | 20 | 0,645–0,93 |
| Peat slabs | 50 | 0,064 |
| Porcelain | 95 | 1,04 |
| Fiber (plates) | 20 | 0,049 |
| Fluorite | 0 | 10,4 |
| Mineral wool | 50 | 0,047 |
| Cinder concrete | 0,93 | |
| Slag wool | 100 | 0,07 |
| Plaster | 20 | 0,779 |
| Cotton (density 81 kg/m3) | 0 | 0,057 |
| Ebonite | 0 | 0,158 |
| Material | T, °С | λ, W/(m K) |
Table 3.7.5
Thermal conductivity coefficients λ (W/(m K)) of some liquid metal coolants
| Coolant | Temperature, °C | ||||||||||
| 0 | 50 | 100 | 150 | 200 | 250 | 300 | 400 | 500 | 600 | 700 | |
| Bismuth (Tm = 271.3 °C; Tbp = 1560 °C) | 14,7 | 15,6 | 16,5 | 17,3 | 18,3 | ||||||
| Potassium (Tm = 63.6 °C; Tbp = 776 °C) | 46,5 | 46,4 | 45,9 | 44,9 | 43,4 | 39,5 | 34,9 | 30,9 | 28,3 | ||
| Lithium (Tm = 180 °C; Tbp = 1350 °C) | 46,1 | 46,3 | 46,6 | 47,1 | 47,6 | 48 | 48,5 | ||||
| Sodium (Tm = 97.8 °C; Tbp = 900 °C) | 86,1 | 84,1 | 81,6 | 78,7 | 75,5 | 68,7 | 63,8 | 60,6 | 59,1 | ||
| Tin (Tm = 231.9 °C; Tbp = 2720 °C) | 30,7 | 31,6 | 33,6 | 35,5 | 37,4 | 39,4 | |||||
| Mercury (Tm = –38.9 °C; Tbp = 356.6 °C) | 7,79 | 8,43 | 9,07 | 9,71 | 10,4 | 11 | 11,6 | 12,6 | 13,3 | ||
| Lead (Tm = 327.3 °C; Tbp = 1751 °C) | 15,1 | 15,5 | 15,9 | 17,7 | |||||||
| Sodium-potassium alloy: 25% Na + 75% K (Tm = 11 °C; Tbp = 784 °C) | 22,7 | 23,3 | 23,8 | 24,5 | 25,1 | 25,8 | 27,1 | 28,4 | 29,7 | 30,9 | |
| Lead-bismuth alloy: 44% Pb + 55.5% Bi (Tm = 123.5 °C; Tbp = 1670 °C) | 11,2 | 11,7 | 12,2 | 12,7 | 13,7 | 14,7 | 15,8 | 16,7 | |||
Table 3.7.6
Thermal conductivity coefficients of pure organic liquids
| Name | Formula | T, °С | λ, W/(m K) |
| Aniline | С6H7N | 16,5 | 0,1774 |
| Acetaldehyde | С2H4O | 21 | 0,1712 |
| Acetone | С3Н6О | 16 | 0,1902 |
| Benzene | C6H6 | 16 | 0,1902 |
| Bromobenzene | С6Н5Br | 20 | 0,1115 |
| 2-Bromobutane | C4H9Br | 12 | 0,1164 |
| 1-Bromopentane | С5H11Br | 18 | 0,0984 |
| 1-Bromopropane | С3Н7Br | 12 | 0,1076 |
| Bromoethane | С2Н5Br | 30 | 0,1198 |
| Butan-1-ol | С4H10O | 20 | 0,1534 |
| Butyl acetate | С6Н12О2 | 20 | 0,1369 |
| Hexane | S6H14 | 30–100 | 0,1376 |
| Hexan-1-ol | С6H14O | 30–100 | 0,1615 |
| Heptane | S7N16 | 30 | 0,1404 |
| Heptan-1-ol | С7H16O | 70–100 | 0,1625 |
| Glycerol | С3Н8О3 | 20 | 0,2943 |
| Dean | S10N22 | 30 | 0,1402 |
| Diisopropyl ether | С6Н14О | 20 | 0,1097 |
| Difluorodichloromethane (Freon-12) | СCl2F2 | 20 | 0,08248 |
| Difluorochloromethane (Freon-22) | CHClF2 | 20 | 0,09295 |
| Dichloromethane (methylene chloride) | CH2Cl2 | 0 | 0,1218 |
| 1,2-Dichloropropane | С3Н6Cl2 | 20–50 | 0,1254 |
| 1,2-Dichloroethane (ethylene chloride) | С2Н4Cl2 | 20 | 0,1264 |
| Diethyl ether | С4Н10О | 30 | 0,1375 |
| N,N-Diethylethanamine (triethylamine) | С6Н15N | 20 | 0,121 |
| Isobutyric acid | С4Н8О2 | 12 | 0,1424 |
| Isopropyl acetate | С5Н10О2 | 20 | 0,1344 |
| 1-Isopropyl-4-methylbenzene (n-cymene) | S10N14 | 30 | 0,1347 |
| 2-Isopropyl-5-methylphenol (thymol) | С10H14O | 13 | 0,1311 |
| Iodobenzene | С6Н5I | 30–100 | 0,1203 |
| 2-Iodobutane | С4Н9I | 12 | 0,08709 |
| 1-Iodopentane | С5H11I | 12 | 0,08499 |
| 1-Iodopropane | С3Н7I | 12 | 0,09211 |
| Iodoethane | С2Н5I | 30 | 0,111 |
| m-Cresol | С7Н8О | 20 | 0,1499 |
| n-Cresol | С7Н8О | 20 | 0,1444 |
| o-Xylene | С8Н10 | –20÷80 | 0,1428 |
| m-Xylene | С8Н10 | 25 | 0,1577 |
| Butyric acid | С4Н8О2 | 12 | 0,1507 |
| Mesitylene | S11N12 | 20 | 0,1359 |
| Methanol | CH4O | 20 | 0,2023 |
| Methyl acetate | С3Н6О2 | 12 | 0,1612 |
| 3-Methylbutan-1-ol | С5H12O | 0 | 0,1478 |
| (3-Methylbutyl)acetate | С7Н14О2 | 20 | 0,1298 |
| 2-Methylpropan-1-ol | С4H10O | 20 | 0,1424 |
| 1-Methyl-3-chlorobenzene | С7Н7Сl | 20 | 0,1298 |
| Methylcyclohexane | S7N14 | 30 | 0,1278 |
| Formic acid | CH2O2 | 12 | 0,2713 |
| Nitrobenzene | С6Н5NO2 | 30–100 | 0,1636 |
| Nitromethane | CH3NO2 | 30 | 0,2153 |
| Nonan | С9Н20 | 30–100 | 0,1413 |
| Nonan-1-ol | С9H20O | 30–100 | 0,1681 |
| Octane | S8N18 | 30 | 0,1452 |
| Octan-1-ol | С8H18O | 30–100 | 0,1663 |
| Oleic acid | С18Н34О2 | 26,5 | 0,2309 |
| Palmitic acid | С16Н32О2 | 72,5 | 0,1715 |
| Pentane | С5H12 | 30 | 0,1349 |
| Pentan-1-ol | С5H12O | 30–100 | 0,1622 |
| Pentachloroethane | С2НCl5 | 20 | 0,1254 |
| Pentyl acetate | С7Н14О2 | 20 | 0,1292 |
| Propane-1,2-diol | С3Н8О2 | 20–80 | 0,2009 |
| Propan-1-ol | С3H8O | 12 | 0,1562 |
| Propan-2-ol | С3H8O | 20 | 0,1408 |
| Prop-2-en-1-ol | С3Н6O | 30 | 0,1798 |
| Propyl acetate | С5Н10О2 | 12 | 0,1369 |
| Propyl formate | С4Н8О2 | 12 | 0,1537 |
| Propionic acid | С3Н6О2 | 12 | 0,1633 |
| Stearic acid | С18Н36О2 | 72,5 | 0,1601 |
| 1,1,2,2-Tetrafluoro-1,2-dichloroethane (Freon-114) | С2Cl2F4 | 30 | 0,0775 |
| Carbon tetrachloride | СCl4 | 20 | 0,1034 |
| 1,1,2,2-Tetrachloroethane | С2H2Cl4 | 20 | 0,1139 |
| Tetrachlorethylene | CCl2=CCl2 | 20 | 0,1619 |
| Toluene | S7N8 | 20 | 0,1349 |
| 1,1,2-Trifluoro-1,2,2-trichloroethane (Freon-113) | С2Cl3F3 | 30 | 0,09085 |
| Trichlorethylene | CHCl=CCl2 | 20 | 0,1162 |
| Acetic acid | С2Н4О2 | 20 | 0,172 |
| Acetic anhydride | С4H6O3 | 21 | 0,2213 |
| Fluorodichloromethane (Freon-21) | CHCl2F | 0,108 | |
| Fluorotrichloromethane (Freon-11) | CCl3F | 20 | 0,09546 |
| Chlorobenzene | C6H5Cl | 30–100 | 0,1447 |
| 2-Chlorobutane | C4H9Cl | 12 | 0,1164 |
| Chloromethane | CH3Cl | –15÷30 | 0,1925 |
| Chloroform | CHCl3 | 20 | 0,103 |
| 1-Chloropentane | С5H11Cl | 12 | 0,1185 |
| 1-Chloropropane | С3Н7Cl | 12 | 0,1185 |
| Ethanol | С2H6O | 20 | 0,1673 |
| Ethyl acetate | С4Н8О2 | 16 | 0,1491 |
| Ethylbenzene | С8Н10 | 20 | 0,1323 |
| Ethylene glycol | С2Н6О2 | 20 | 0,2611 |
| Name | Formula | T, °С | λ, W/(m K) |
Table 3.7.7
Thermal conductivity coefficients λ (W/(m K)) of some refrigerants in the liquid state
| Refrigerant | Formula | Temperature, °C | ||||||
| –30 | –20 | –10 | 0 | 10 | 20 | 30 | ||
| Ammonia | NH3 | 0,57 | 0,57 | 0,558 | 0,547 | 0,518 | ||
| Difluorodichloromethane (Freon-12) | СCl2F2 | 0,106 | 0,101 | 0,097 | 0,092 | 0,087 | 0,083 | 0,078 |
| Sulfur dioxide | SO2 | 0,223 | 0,207 | 0,212 | 0,205 | 0,199 | 0,193 | |
| Carbon dioxide | CO2 | 0,151 | 0,14 | 0,128 | 0,116 | 0,093 | 0,07 | |
| Fluorotrichloromethane (Freon-11) | CCl3F | 0,12 | 0,115 | 0,11 | 0,106 | 0,101 | 0,095 | 0,091 |
| Chloromethane | CH3Cl | 0,188 | 0,179 | 0,171 | 0,162 | 0,154 | ||