Iron and steel are the materials from which the skeleton of modern technological civilization is made. But - alas! — and the steel foundation of our civilization has its own weak spot. It is called corrosion, from which iron and steel do not have the natural protection that the oxide film provides for many non-ferrous metals - tin, zinc or aluminum.
Steel needs protection from corrosion - and the best protection for it is galvanizing. (or galvanized). Galvanized sheets will last much longer than regular steel sheets.
Why zinc?
First of all, because zinc is quite common and inexpensive, it itself is practically resistant to corrosion, melts at a not very high temperature (about 420 degrees, which is much lower than the melting point of iron), and has acceptable strength. And at the same time - what is important! - has a stationary electric potential of -0.76 V, that is, much more negative than that of iron.
Due to this property, zinc, even if the integrity of the coating is damaged and under the influence of electrolytes, will play the role of an anode in the resulting electrochemical reactions. That is, it will slowly dissolve, thereby protecting the steel base of the structure from electrochemical corrosion. In general - give us zinc coatings! Just in what ways to apply them? This is a rather interesting question, since there are several such methods - and each of them has its own pros and cons. Let's consider them...
Galvanizing on hangers
However, the method of galvanizing fasteners described above is not always acceptable. As the workpieces move inside the drum, they inevitably come into contact with each other. There is a danger of their abrasion. What will be the value of protective coating, galvanizing, if the parts themselves are mechanically damaged? In this case, galvanizing on hangers is used as an alternative method. It will be appropriate when:
- external threads are applied to the parts,
- increased demands are placed on their strength characteristics,
- it is necessary to achieve maximum coating uniformity and corrosion resistance;
- The attractive appearance of the products is extremely important to the customer.
Hot galvanizing
This is, at first glance, the simplest and most reliable method of creating a zinc film on cast iron and steel products: these objects are simply immersed in molten zinc, and then taken out already coated with a layer of zinc 40 to 80 microns thick - that is, quite dense and wear-resistant.
However, not everything is so simple: in order for the zinc film to reliably “catch” on the surface of ferrous metal, this surface must be thoroughly cleaned and fluxed (that is, coated with a composition that should prevent it from oxidizing before contact with molten zinc and ensure reliable adhesion zinc film).
It should be borne in mind that molten zinc hardens quite quickly and can therefore form sagging up to 1 mm. thick, which is undesirable in cases where there is a thread on the surface of galvanized products. In addition, the technology itself imposes restrictions on the size of the products that we want to galvanize in this way - they cannot be larger than baths of molten zinc (and they cannot be very large by definition).
Galvanizing at home
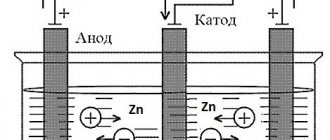
The technological process of galvanizing involves the deposition of metal cations on the anode. A similar chemical reaction occurs in a bath of electrolyte when exposed to electric current.
Where to find electrolyte
Any solution of zinc salts can be used as an electrolyte. The most popular and easily accessible are zinc chloride and hydrochloric acid. Also, an electrolyte with the necessary properties can be obtained by etching zinc in sulfuric acid. This reaction must be carried out very carefully. It is accompanied by the release of a large amount of thermal energy and explosive hydrogen.
Pickling of zinc in sulfuric acid with the release of hydrogen and obtaining zinc salts
How to get zinc
To galvanize at home, you need to prepare zinc, which can be obtained in the following ways:
- using regular salt batteries;
- fuses from the times of the Soviet Union;
- any parts with zinc coating;
- pure metal, which can be found in appropriate stores where chemical reagents are sold.
Scheme for obtaining zinc from batteries
Preparing for the procedure
To create a high-quality metal coating, several preparatory operations should be performed:
Galvanizing process
- prepare a galvanic bath. Any glass or plastic container can play its role;
- install stands for the anode and cathode;
- the electrolyte should not contain undissolved salt crystals , for which distilled water is additionally introduced;
- The role of the anode is performed by a zinc plate. The larger its area, the better the quality of the coating;
- The plus from the power source is connected to the anode. There can be several of these elements if desired;
- A minus is connected to the cathode. Zinc particles will be deposited on its surface;
- The cathode must be cleaned of rust and any contaminants. Before processing, it is additionally dipped in an acid solution;
- the cathode must be at the same distance from the anode to ensure uniform coverage on all sides;
- with a constant current output is used as a power source
- the greater the current and voltage, the faster the reaction will occur and the looser the protective film will be;
- when using a car battery, an incandescent light bulb of up to 20 W is included in the circuit to reduce the current.
Device for galvanizing at home
Technology for creating zinc film
To create a high-quality protective coating on the metal surface, after the preparatory operations have been carried out, the current source is connected to the network, and the cathode is dipped into a galvanic bath. This process should take place without violent boiling. If this is observed, you may suspect too much current in the system. To reduce it, several additional consumers are connected to the electrical circuit.
Gradually, a metal coating will form on the surface of the cathode. The longer this process takes place, the thicker the protective layer on the metal will be.
Cold galvanizing method
Unlike hot galvanizing, its cold version has nothing to do with the size of the parts and surfaces being galvanized, since it consists of applying electrolytic solutions of zinc in various volatile liquids to them. Such solutions (galvanol, cynotane, cynotern, zincnol) are applied to the steel surface from a spray bottle: the solvent dries, but the zinc coating remains - and it “sits” on the steel surface very reliably, with high adhesion. In this case, the surface to be coated does not need to be fluxed; it can simply be cleaned of rust and dirt.
The cold galvanizing method is good for its simplicity
and suitability for outdoor work. But the appearance of steel coated in this way will simply be a matte gray without a metallic sheen.
Therefore, cold galvanizing is best used as a “paintable coating”.
How to distinguish galvanized metal from regular metal
Over time, the zinc layer becomes dull, and it becomes difficult to visually distinguish it from unprotected steel.
Let's say you want to buy galvanized, but not new, although high-quality rolled steel. How to determine that it has really been galvanized? The easiest way is to hold a magnet to the metal. Unprotected steel will exhibit a magnetic effect, but zinc, known for its non-magnetic properties, will be indifferent to this experience. Finally, you can check the galvanization of a part in another simple way, although it will probably surprise the seller: you can lick the surface of the metal. Galvanization has a pronounced chalky taste.
We have described only the simplest and “instant” ways to check for galvanization. Others require time or the use of laboratory equipment.
Galvanic galvanizing method
But the galvanic galvanizing method gives the galvanized surface an extremely attractive appearance.
It consists in the fact that current flows through a bath of electrolyte, with zinc plates serving as the anode and steel products as the cathode. Under the influence of current, zinc dissolves in the electrolyte, and its ions are deposited on the iron. The result is a thin (from 4 to 20 microns) film that not only protects the steel from corrosion, but also gives the surface an aesthetic appearance. The surface coated with galvanic zinc can acquire (depending on its thickness) a blue-blue, light gray or matte white metallic sheen. The main advantages of such a coating will be the uniformity of its thickness over the entire surface to be coated.
But these advantages also come with their own disadvantages: a thin and beautiful galvanic coating will not be resistant to abrasion, and an increase in its thickness will be fraught with the fact that the steel during the galvanizing process can also acquire the so-called. "hydrogen fragility". However, galvanic galvanizing. Due to its low cost, it is often used for anti-corrosion protection of various types of fasteners, metal products and decorative elements.
Why is metal galvanized?
Steel products are known to be very vulnerable to corrosion, especially when used in damp environments. Galvanizing steel gives this metal reliable protection from destruction. Zinc, reacting with steel, forms a galvanic couple with it and acquires a greater degree of electronegativity than steel.
It is zinc that takes the first blow from aggressive factors, while steel does not react and remains protected by the zinc coating. Accordingly, the anti-corrosion protection will remain in effect until the galvanization is completely destroyed. But even in those places on the steel surface where it wears off, zinc reacts with oxygen and water to form zinc hydroxide. This connection also does a good job of protecting the surface of the steel product from corrosion.
Thermal diffusion galvanizing
The main disadvantage of galvanic galvanizing, “hydrogen embrittlement,” is avoided by thermal diffusion galvanizing technology.
It is based on the fact that zinc, under certain conditions, can evaporate from the surface of a zinc-containing powder and penetrate into the surface layers of iron, resulting in the formation of a complex zinc-iron alloy.
Such diffusion becomes possible when
high (from 290 to 400 degrees) temperature and the presence of electrical potential, at which steel products act as an anode.
The thermal diffusion process is carried out in a rotating container at reduced pressure (0.1 atmosphere) in a reducing hydrogen atmosphere. Thermal diffusion galvanizing of each batch of fastening hardware requires from 90 to 180 minutes.
As a result, these parts will acquire a mouse-gray color - but with it, increased surface strength and excellent corrosion resistance (3-5 times better than with galvanic galvanizing and one and a half to two times better than with hot-dip galvanizing).
In this case, the uniformity of the coating will be ideal, and there is no point in talking about the adhesion of such a coating - it simply “merges” with the ferrous metal, so it is simply impossible to separate it from it. Of course, the appearance of hardware galvanized in this way does not bring much aesthetic pleasure, but bolts, nuts, springs and screws are not required to be particularly beautiful.
Thermal-diffusion galvanizing has only one drawback, but it is significant - due to the nature of the technology, it can only be used for small-sized objects.
Metal galvanizing methods
There are the following methods of galvanizing metal:
- cold;
- hot;
- galvanic;
- thermal diffusion;
- gas-thermal.
You need to choose one or another method for galvanizing steel parts or structures depending on the conditions of use and the characteristics of the protective layer. Regardless of the galvanizing technology used, it is necessary to determine the thickness of the protective layer. It depends on parameters such as the period of exposure of the working environment to the metal and the processing temperature.
When using steel structures on the surface of which a layer of zinc is applied, you must remember that they cannot be subjected to strong mechanical stress, since the protective metal coating is highly brittle and can be destroyed.
Let's look at different types of metal galvanizing.
Hot galvanizing method
Hot galvanizing of metal allows us to achieve maximum quality of products and ensure their durability. This method has a key drawback - its implementation involves the use of chemical reagents to treat the surface, and the procedure is performed in molten zinc.
The hot galvanizing method of steel includes preparing the surface of the product and the actual procedure of coating the metal with zinc.
Steps to prepare the surface to be treated:
- cleansing;
- degreasing;
- application of acid solutions;
- washing and fluxing;
- drying the surface.
When the surface goes through all the stages of preparation and dries, the product is placed in a special bath with molten zinc. A thin layer of zinc and iron is formed on the surface of the steel, which reliably provides anti-corrosion protection. When the product is removed from the bath, it is blown with compressed air to dry it and remove excess zinc from the surface.
The disadvantage of this method is that the dimensions of the products are limited by the dimensions of the bath with molten zinc. This method is practiced at large production facilities when working with power transmission poles, scaffolding or lighting masts.
The method involves a lot of labor and the use of complex equipment, so it is not suitable for home use.
Cold galvanizing of steel
This method of processing metal with zinc has gained wide popularity in recent years. The main reason is the high manufacturability and simplicity of the method, coupled with the high protective properties of the layer on the metal surface. The option is suitable for galvanizing metal with your own hands, since no special equipment is required for the work.
Cold galvanizing technology involves applying a zinc-containing mixture, for example, zinconol, to the surface of the product. It must be applied with a roller or brush. If you need to cover structures of complex shapes or hard-to-reach places, you can use a spray gun. Thanks to special compounds, a protective layer is formed on the surface, consisting of more than 90 percent zinc.
This galvanizing method is the only one acceptable for providing anti-corrosion protection to structures that cannot be galvanized in any other way. For example:
- installed pipes;
- elements of railway tracks;
- power line supports and other structures in a stationary or mounted version.
Zinconol and other cold galvanizing compounds are used during repairs if there is a need to restore the damaged zinc layer on metal structures. For example, this is relevant for the purpose of restoring the galvanization of a car body.
Cold galvanizing of steel products is carried out over a wide temperature range; the resulting coating has high protection, elasticity, and resistance to mechanical stress and temperature changes.
Cold galvanizing has its disadvantages. For example, the formed coating does not have high enough resistance to mechanical stress; safety precautions must also be strictly observed if the procedure involves the use of organic solvents.
Galvanic galvanizing method
During galvanic galvanizing, an electrochemical effect is applied to the surface of the product. The resulting coatings have high thickness accuracy and exceptional smoothness. Also, a protective layer with a thickness of about 20–30 microns is formed on the metal surface.
With the help of such galvanizing, the thickness of the protective layer can be adjusted, while the layer is uniform and has high decorative qualities. Metal and zinc are combined at the molecular level during galvanizing; the coating has high adhesion to the base material. The degree of adhesion is affected by the presence of oxide and fatty films on the surface, which are almost impossible to remove.
Galvanic galvanizing is carried out as follows:
- the structure and zinc plates are placed in an electrolytic solution, then the positive and negative contacts of the current source are connected to the wall of the bath and the plates;
- due to the potential difference, the plates dissolve in the electrolyte, and zinc molecules settle on the surface of the product and form a uniform protective layer.
The key advantage of this method is that in this way a protective layer is formed on the surface, which has special decorative characteristics. The thickness of the layer can be adjusted. But the method also has its drawbacks. In particular, this is a high cost.
Thermal diffusion galvanizing technology
This technology is also called sherardization. It was developed in the 20s of the last century, but for a long time it was not widely used. It was only in the 90s that the method gained popularity.
The essence of the method is that the part, together with a dry zinc-based mixture, is placed in a sealed container, where a temperature of about 2600 degrees is created. At this temperature, zinc goes into a gaseous state, and accordingly, the process of diffuse penetration of zinc atoms into the surface layer of the product is accelerated. This galvanizing technology is used in cases where it is necessary to form a protective layer with a thickness of 15 microns on the metal surface.
The preparation of metal products for thermal diffusion coating with zinc does not differ from the hot method. Advantages of this method:
- the process takes place in a sealed container, therefore, it is environmentally friendly;
- there are almost completely no pores on the protective coating, which has high adhesion to the surface;
- the coating receives a high degree of protection;
- complex geometric shapes and parameters of zinc-coated products are preserved;
- the resulting waste does not require special disposal.
Disadvantages of the thermal diffusion method:
- the finished coating does not have a metallic sheen and has a dirty gray tint;
- low productivity;
- zinc dust in the air during work can harm the body;
- The zinc coating is not uniform in thickness.
Thermal spraying
The gas-thermal galvanizing method is suitable for coating a three-dimensional part or sheet of metal with zinc. Its essence is that zinc, in the form of a dry mixture or wire, is sprayed in a gaseous environment onto the surface of the workpiece. The technology is used to apply a layer of zinc to large-sized products that cannot be processed otherwise.
Zinc plating process:
- particles of molten metal are applied to the surface to be treated, forming a thin layer with a scaly structure;
- Paints and varnishes are applied to the porous coating. The layer created by combining protects the product and allows it to be used for a long time in conditions of high humidity, constant exposure to fresh or sea water and other aggressive environments.
The parameters of zinc coatings applied by the methods listed above are established by the relevant GOST.
Electrochemical galvanizing
Using this technology for processing small products, it is possible to obtain a coating whose thickness does not exceed 10 - 15 microns. However, this thickness of the protective layer is not sufficient for the parts to be used for external fastening.
Of course, the technique makes it possible to create coatings of greater thickness, but this requires increased electrical energy consumption. It is also worth considering that waste solutions contain complexons, which complicate the process of processing solutions.
Sherardization technology (thermal diffusion galvanizing) allows you to create uniform protective coatings of sufficient thickness (25 - 30 microns), eliminating the possibility of parts sticking together. However, this processing method also involves increased consumption of thermal energy required to heat the equipment, as well as regular repairs of work boxes.
In addition, the relatively low level of use of zinc powder does not allow the use of technology to provide reliable anti-corrosion protection of fastening elements.
Order metal galvanization from Tochinvest Zinc
When contacting our company, all clients receive the following benefits:
- The capacity of our company reaches 120,000 tons per year, which allows us to quickly carry out work in compliance with the agreed deadlines.
- The company is equipped with 3 production workshops for hot-dip galvanizing of small parts and large structures.
- We are the only company that has the deepest bathtub in the Central Federal District - 3.43 m.
- The coating using hot-dip galvanizing technology is applied taking into account the requirements regulated by GOST 9.307-89.
- Since 2007, only high-tech equipment from the German-Austrian company KVK KOERNER and the Czech company EKOMOR has been used in production.
You can find out more about the cost of processing and the timing of the work by calling the numbers listed on the website.
Call! Return to articles Share article