Scope of gas application
Propane-butane is a unique gas-based substance that contains molecules of the same name.
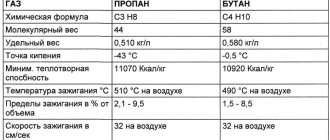
The generally accepted chemical formula of propane consists of molecules and atoms of two main components - propane (C3H8) and butane (C4H10).
Widely used for domestic purposes, this gas is used almost everywhere - from cooking food in a frying pan, to cutting a thick layer of metal, to its active use in various industries in general.
It is also increasingly used by people who have given up gasoline-based fuel to fuel their cars.
Domestic gas explosion prevention
Knowing why gas most often explodes in apartments, you can draw up a list of actions to prevent accidents.
It will include:
- operation of authorized equipment that is within its expiration date;
- connection and installation performed by licensed professionals;
- proper storage and installation of cylinders;
- coordination of all project changes with the gas service;
- regular inspection of the gas pipeline, connections and equipment by gas workers;
- maintaining the ventilation system in working order;
- cooking on a gas stove in the constant presence of capable adults;
- use the equipment strictly for its intended purpose.
In addition, it is important to listen to odors and monitor the tightness of valves, taps, and flange connections. At the slightest suspicion, contact the gas service that supplies fuel.
Unfortunately, reviews from people living in gasified houses contain many messages about a formal approach to servicing gas equipment. To avoid this, you should look at the list of mandatory works and make sure that all declared activities have been carried out in full
Since apartment buildings make residents dependent on each other, sometimes you have to look after more than just your own gas equipment. If neighbors violate safety regulations, you must write a statement to the gas service.
It must indicate the exact address, the reason for the appeal, the names of the residents who signed the application, information about the violators, and the date of recording. The reason may be the presence of prohibited equipment, violation of operating rules, or the smell of gas.
Chemical and physical properties
Propane-butane has unique chemical and physical properties, which literally makes it so popular among consumers all over the world.
Firstly, this representative of liquefied carbon gases remains in liquid form exclusively at high pressure, which is equal to 16 atmospheres. Therefore, during transportation, the substance is transported only in gas cylinders with appropriate pressure.
The combustion temperature of propane is not equal to any specific number and ranges between 800-1970 degrees Celsius. Such high indicators fully justify the benefits that it brings to human life, because the combustion of this mixture has high efficiency when performing any tasks related to the use of this gas.
The boiling point of propane is -42 degrees Celsius, which indicates a guarantee of safe operation under normal conditions.
But since we are considering a mixture of propane and butane, this figure can rise to -25 degrees and even higher, depending on the percentage of components in the substance. It is worth considering that propane freezes at a temperature of -188 degrees.
When transporting the substance, do not forget about the temperature of the propane in the cylinder, which should not exceed 15 degrees Celsius.
This approach is considered the safest, since when transporting gas cylinders at higher temperatures, the risk of fire increases significantly.
By the way, as for the ignition temperature of propane-butane, here they differ - for the first it is 504 degrees Celsius, and for the second - 430. But, despite such a large number of differences between its components, this representative of liquefied carbon gases is quite competitive with gasoline combustible mixtures.
Propane , C3H8, is an organic substance of the alkanes class. Contained in natural gas, it is formed during the cracking of petroleum products, during the separation of associated petroleum gas, “wet” natural gas, as a by-product during various chemical reactions. The flammable hydrocarbon gas liquefied under pressure in a cylinder, colloquially referred to as “Propane” in accordance with the inscription on the cylinder, is actually a mixture of propane and butane. The correct name of this gas is SPBT - a technical propane-butane mixture.
As a representative of hydrocarbon gases, it is fire and explosive. Low toxicity, but has a harmful effect on the central nervous system (has weak narcotic properties). Colorless, odorless gas. Very slightly soluble in water. Boiling point −42.1 °C. Freezing point -188 °C. Forms explosive mixtures with air at vapor concentrations from 2.1 to 9.5%. The auto-ignition temperature of propane in air at a pressure of 0.1 MPa (760 mm Hg) is 466 °C. The critical temperature of propane is Tcr = 370 K, the critical pressure Pcr = 4.27 MPa, the critical specific volume Vcr = 0.0444 m3/kg. The density of compressed and liquefied propane at 298 K is 0.493 kg/l. Liquid phase density = 510 kg/m3.
Butane (C4H10) is an organic compound of the alkanes class. In chemistry, the name is used primarily to refer to n-butane. The mixture of n-butane and its isomer isobutane CH(CH3)3 has the same name. The name comes from the root “but-” (the English name for butyric acid is butyric acid) and the suffix “-an” (belonging to alkanes). In high concentrations it is poisonous; inhalation of butane causes dysfunction of the pulmonary-respiratory system. Contained in natural gas, it is formed during the cracking of petroleum products, during the separation of associated petroleum gas, and “wet” natural gas. As a representative of hydrocarbon gases, it is fire and explosive, low-toxic, has a specific characteristic odor, and has narcotic properties. In terms of the degree of impact on the body, the gas belongs to substances of the 4th hazard class (low-hazard) according to GOST 12.1.007-76. Harmful effect on the nervous system. Butane is a colorless flammable gas with a specific odor, easily liquefies at normal pressure from −0.5 °C, freezes at −138 °C; at elevated pressure and normal temperature it is a highly volatile liquid. Critical temperature +152 °C, critical pressure 3.797 MPa. Solubility in water - 6.1 mg in 100 ml (for n-butane, at 20 °C), much better soluble in organic solvents). It can form an azeotropic mixture with water at a temperature of about 100 °C and a pressure of 10 atm. The density of the liquid phase is 580 kg/m3.
Natural and liquefied hydrocarbon gases used for municipal and domestic needs are odorless. Therefore, to ensure safety when working with household gas, it is necessary to add special substances - odorants - to their contents. The introduction of odorants makes it possible to detect leaks in gas communications and apparatus, as well as the presence of gases in industrial and residential premises long before the formation of their explosive or toxic concentrations and thereby increases the safety of gas use. Ethyl mercaptan is usually used for odorization of gases; norms: 16 mg per 1 m3 natural. gas; 60 g per 1 ton of liquid product (if the liquefied gas contains propane up to 60%, butane, etc. more than 40%), 90 g per 1 ton (propane over 60%, butane, etc. up to 40%). The degree of odorization is controlled by chemical, physical-chemical. and organoleptic. methods.
The importance of SPBT gas both in everyday life and in industry cannot be overestimated. SPBT is used:
When performing gas-flame work in factories and enterprises:
- in procurement production;
- for cutting scrap metal;
- for welding non-critical metal structures.
- During roofing work.
- For heating industrial premises in construction.
- For heating industrial premises (on farms, poultry farms, in greenhouses).
- For gas stoves, water heaters in the food industry.
At home:
- when preparing food at home and on the go;
- for heating water;
- for seasonal heating of remote premises - private houses, hotels, farms;
- for welding pipes, greenhouses, garages and other utility structures using gas welding stations.
- Recently, it has been widely used as automobile fuel, as it is cheaper and more environmentally friendly than gasoline.
SPBT is stored and transported in a liquid state under pressure in tanks or metal cylinders of bright red color (sometimes polymer-composite cylinders) with a capacity of 5,12, 27 and 50 liters. According to GOST 20448-90, the propane component in the cylinder should not be less than 40%. A more precise specific proportion of propane-butane is selected by the supplier depending on the expected ambient temperature. Very often, unscrupulous suppliers, using the rather broad framework of GOST, force the consumer to buy gas with a high butane content for winter, which, due to the low temperature, does not completely evaporate from the cylinder. Refilling such a cylinder is much cheaper, which means more profit. To control the quality of the gas proportion, the consumer must (with mandatory observance of safety measures) try to pour a small amount of gas from the cylinder onto a newspaper on the street at the temperature at which the cylinder is supposed to operate. The gas from the newspaper should evaporate within 5-10 minutes, leaving no oil stains on it. Otherwise, the gas mixture will not be able to completely evaporate from the cylinder and the cylinder will not be used properly. Such a consumer must either negotiate with the supplier to change the proportion of propane-butane in the cylinder, or switch to PT gas (technical propane), which is much more expensive. It is not recommended to use gas purchased at gas stations for industrial work due to the fact that organizations involved in the sale of liquefied hydrocarbon gases use cheaper BT gas (technical butane) to refuel cars, which is actively mixed in the cylinder while the car is moving and heated in the gas gearbox located directly on the car engine. It is not possible to use BT for working with metal at air temperatures starting from +10 degrees Celsius and below without additional heating equipment.
A standard 50-liter cylinder is filled with 40 liters of SPBT gas, which is approximately 21 kg. The gas in the cylinder is in the “dew point” state, that is, under a pressure (16 atmospheres) at which it condenses at a given temperature. In this state of gas, the pressure in the cylinder remains constant until it completely evaporates (this is why an industrial propane reducer has only one pressure gauge), so the fullness of the cylinder can only be checked by placing it on a scale. During operation, it is advisable to artificially increase the gas evaporation mirror in the cylinder (especially at sub-zero temperatures), tilting it slightly when placing it on its side, since the evaporation process is endothermic, that is, it occurs with the absorption of heat and, consequently, a decrease in temperature. An even deeper aggravation of the process of freezing of the cylinder is caused by attempts to use it without a reducer - for work that requires greater gas consumption. In this case, the process of adiabatic expansion - throttling - takes place. To eliminate these problems, gas cylinders equipped with reducers must be combined into blocks of cylinders connected in parallel. It is strictly forbidden to heat SPBT gas cylinders with open fire. If the cylinder valve freezes and the gas supply stops, it is recommended to take the cylinder into a room with a positive temperature for a while and then adjust the gas supply with the reducer to characteristics that prevent freezing. The exact data on gas cylinders is given in the table.
| 50 liter cylinder | 27 liter cylinder | 27 liter cylinder, cap | 12 liter bottle | 12 liter cylinder, cap | 5 liter bottle | 5 liter cylinder, cap | |
| Working pressure, MPa | 1,6 | 1,6 | 1,6 | 1,6 | 1,6 | 1,6 | 1,6 |
| Test pressure, MPa | 2,5 | 2,5 | 2,5 | 2,5 | 2,5 | 2,5 | 2,5 |
| Breaking pressure, MPa, not less | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Volume l not less | 50 | 27 | 27 | 12 | 12 | 5 | 5 |
| Weight of propane, kg no more | 21,2 | 11,4 | 11,4 | 5 | 5 | 2 | 2 |
| Empty cylinder weight, kg | 22,0±2,2 | 14,5±1,4 | 14,5±1,4 | 6,0±0,6 | 6,0±0,6 | 4,0±0,4 | 4,0±0,4 |
| Wall thickness of the cylinder body, mm | 3 | 3 | 3 | 2 | 2 | 2 | 2 |
| Height, mm no more | 1015 | 590 | 590 | 485 | 485 | 295 | 295 |
| Diameter, mm | 299+3,0 | 299+3,0 | 299+3,0 | 222+2,0 | 222+2,0 | 222+2,0 | 222+2,0 |
| Operating temperature, °C | -40 to +45 | -40 to +45 | -40 to +45 | -40 to +45 | -40 to +45 | -40 to +45 | -40 to +45 |
| Locking device on the gas cylinder | Valve VB-2 GOST 21804-94 | Valve KB-2 or Valve VB-2 GOST 21804-94 | Valve VB-2 GOST 21804-94 | Valve KB-2 or Valve VB-2 GOST 21804-94 | Valve VB-2 GOST 21804-94 | Valve KB-2 or Valve VB-2 GOST 21804-94 | Valve VB-2 GOST 21804-94 |
Basic physical and chemical properties of LPG components and their combustion products
The main characteristics of LPG include:
- evaporation/condensation temperature;
- ignition temperature;
- heat of combustion;
- density;
- volume expansion.
Important characteristics are the explosive limits when mixed with air, the speed of fire spread during combustion, and the conditions for complete combustion.
Evaporation/condensation temperature
At normal pressure is:
- for propane – minus 42 °C;
- for butane – minus 0.5 °C.
If the temperature of gases rises above these values, they begin to evaporate; when they fall below, they begin to condense. As a rule, liquefied gas is supplied in the form of a mixture (butane + propane). Therefore, the actual evaporation/condensation temperature depends on their ratio.
Propane-butane gas poisoning
Liquefied propane-butane gas, as well as the product of its incomplete combustion, carbon monoxide, is toxic and can cause suffocation or severe poisoning with fatal consequences. With mild and moderate carbon monoxide poisoning, headaches (mainly in the temples), dizziness, nausea, vomiting, severe weakness in the arms and legs, palpitations appear, and in severe cases, a darkened consciousness, often an excited state with erratic movements or loss of consciousness.
Gas leakage occurs as a result of a breakdown of the hose connecting the gas pipeline to the stove, depressurization of threaded connections, forgetfulness of people, pranks of children, or dousing the flame with water. If you smell gas in the room, immediately turn off the gas supply to the stove. At the same time, do not light matches, do not turn on the lights and electrical appliances (it is best to turn off the power to the entire apartment by turning off the power supply at the distribution panel) so that a spark cannot ignite the gas accumulated in the apartment and cause an explosion. Call the emergency gas service (telephone “04”). Thoroughly ventilate the entire apartment, not just the gas-filled room, by opening all windows and doors. Leave the room and do not enter it until the smell of gas disappears.
If others show signs of gas poisoning, take them out into the fresh air and place them so that their head is higher than their feet. Until emergency services arrive, do not bring open flames or turn electrical appliances on or off.
Specifications
To the question: “How are the chemical and physical properties related to the technical features of this mixture?”, it is worth considering all possible answers.
- Firstly, due to its high “holding” pressure in the liquid state, this gas is too inert. That is, it can easily be converted from a liquid to a gaseous state. This is a very useful feature in industries where this is absolutely necessary.
- Secondly, the low boiling and freezing points make the propane-butane mixture resistant to “collisions” with substances of nitrogen origin. Consequently, it guarantees its safety from freezing and boiling.
- And, of course, it is worth noting the high combustion temperature of propane, without which its benefits would not be so significant for achieving certain household or industrial goals.
If the explosion has already occurred
The algorithm of actions in the event of an explosion is identical. If possible, turn off the valves and leave the room. When calling, you should indicate the fact of an explosion and seek help from doctors, firefighters and gas workers. The call can be made by calling 112.
According to the observations of rescuers, panic among residents becomes a significant obstacle to the coordinated actions of emergency services. Therefore, during rescue operations, it is advisable to calm down and strictly follow the instructions of the specialists.
In 90 percent of cases, a gas explosion is accompanied by a fire. Therefore, the actions in such an accident are identical to rescue from fire.
To do this you need:
- Press a damp cloth bandage to your face and breathe only through it.
- Determine the path to the exit. If it is blocked by local fires, try to neutralize them by throwing a blanket or thick outerwear over the fireplace.
- If it is not possible to exit through the main entrance, you should evaluate the chances of leaving the premises through the windows.
In some situations, it is wiser to wait for rescuers rather than evacuate yourself. Therefore, it is important to correctly assess the risks and not give in to panic.
When leaving the apartment after the explosion, you must not use the elevator. The mine quickly fills with smoke, equipment stops functioning and gets stuck between floors
After leaving the house, rescuers advise moving away from the building as far as possible and staying at a safe distance until all sources of danger have been eliminated. Emergency services personnel will notify residents of this.
Conclusions and useful video on the topic
In the video, the specialist once again lists the main causes of accidents, mentions the need for constant monitoring of the gas pipeline, and shows samples of automatic gas analyzers:
Can a propane tank explode? Yes! Can natural gas explode in an apartment? Yes. Any fuel is a source of danger. Therefore, it is mandatory to comply with all rules and regulations for the storage, installation and operation of gas equipment.
If you have useful information, want to share your experience or warn other users, write. Below is a form where anyone can ask questions, post comments or photos. Participate in discussions and express your competent opinion.
Procedure in case of explosion threat
An incorrectly opened or dropped cylinder will leave no choice - the substance will detonate instantly. And when a leak is detected, there is almost always time to neutralize the gas. Leaks can be detected in four ways: through gas analyzers, by soaping the joints, by the appearance of an odor, or by feeling the emanating cold from the joints.
In all cases it is necessary:
- Organize intensive ventilation.
- Close the gas valve.
- Remove people and animals from the premises and warn neighbors.
- Contact the gas service emergency department.
It’s better to call rescuers as soon as you go outside. Before the specialists arrive, it is advisable to monitor the entrance to the house so that no one can enter the polluted apartments.
To quickly navigate in emergency situations, you should know in advance the features of dialing emergency numbers of your mobile operator. Subscribers of Megafon, MTS and TELE2 can call the gas service by dialing 040. When using Beeline services, you need to dial 004, while being served by Motiv - 104
The main thing is to leave the room quickly and without panic if a leak is detected. Therefore, if you find difficulties finding taps or opening windows, it is better to leave everything as it is and go outside.