In laboratory conditions, it is possible to achieve a colorless fire, which can be determined only by the vibration of the air in the combustion area. Household fire is always “colored”. The color of a fire is determined primarily by the temperature of the flame and what chemicals it burns. The high temperature of the flame allows atoms to jump to a higher energy state for some time. When the atoms return to their original state, they emit light at a specific wavelength. It corresponds to the structure of the electronic shells of a given element.
Famous blue
the glow that can be seen when natural gas burns is caused by carbon monoxide, which gives this shade. Carbon monoxide, a molecule made up of one oxygen atom and one carbon atom, is a byproduct of the combustion of natural gas.
Try sprinkling a little table salt on the gas stove burner - yellow tongues will appear in the flame. Such a yellow-orange flame
give sodium salts (and table salt, remember, is sodium chloride). Wood is rich in such salts, so an ordinary forest fire or household matches burn with a yellow flame.
Copper makes the flame green
shade. With a high copper content in the combustible substance, the flame has a bright green color, almost identical to white.
Barium, molybdenum, phosphorus, and antimony also give green color and its shades to fire. In blue
Selenium colors the flame, and boron colors
the flame blue-green
. A red flame will give lithium, strontium and calcium, a purple flame will give potassium, and a yellow-orange hue will come out when sodium is burned.
Flame temperature when burning certain substances:
| Match | 750-1200°С | |
| Natural gas | 400-800°С | |
| Alcohol | 900°С | |
| Kerosene | 1100°С | |
| Petrol | 1300-1400°С | |
| Magnesium | 2200°C |
Do you know?
Due to the property of atoms and molecules to emit light of a certain color, a method has been developed for determining the composition of substances, which is called spectral analysis
. Scientists study the spectrum that a substance emits, for example, when it burns, compare it with the spectra of known elements, and thus determine its composition.
Your comments:
At least I looked at Wikipedia and didn’t embarrass myself
Source: allforchildren.ru
A flame is a gaseous medium in which the interaction of fuel and oxidizer occurs, heat is released and high temperatures develop.
How to disable?
The gas control function is undoubtedly a very important part for a cooker. Its main advantages are described below.
- Preventing gas leaks – ensuring fire and explosion safety. In different models, the fuel supply shutdown time is not the same: on average it is 60-90 seconds.
- Since gas delivery stops even if the handle is released prematurely, this provides protection for children. As a rule, the child is not able to hold the button long enough for the gas to turn on.
- There is no need to constantly monitor the preparation of the dish. This mode is intended for stoves with electric ignition.
But when turning on a stove with automatic ignition, its handle must be held for some time in order for the flame to ignite. This happens because the thermocouple must heat up before the gas enters the system and the fire ignites.
This time period is different for each manufacturer. For brands such as Darina or Gefest, the waiting time is up to 15 seconds. For Gorenje models, the mechanism operates after 20 seconds. Hansa works faster: the fire is ignited in just 10 seconds.
If you have experience working with similar devices, and their structure is familiar, then you can do this yourself. First of all, you must turn off the gas supply. Then you should open the gas control system, disconnect the thermocouple and remove the solenoid valve.
After this, you need to disconnect the spring from it - the main element that brings the faucet into tone. Then you need to reassemble the mechanism and return it back.
The manipulation is not difficult, but you need to be aware that the work is being carried out with an explosive device. In addition, the regulatory authority may impose a fine if such unauthorized behavior is detected.
If this function is useless for the user, and he is determined to disable it, then it is necessary to call a specialist. After disconnecting, the controller will make an appropriate entry in the device’s operating book, indicating the date and reason for canceling the function.
Temperature indicators
Each fire zone of a candle or burner has its own values, determined by the supply of oxygen molecules. The temperature of the open flame in its different parts ranges from 300 °C to 1600 °C.
An example is a diffusion and laminar flame, which is formed by three shells. Its cone consists of a dark area with a temperature of up to 360 °C and a lack of oxidizing substances. Above it is a glow zone. Its temperature ranges from 550 to 850 °C, which promotes thermal decomposition of the combustible mixture and its combustion.
The outer area is barely noticeable. In it, the flame temperature reaches 1560 °C, which is due to the natural characteristics of fuel molecules and the speed of entry of the oxidizing substance. This is where the combustion is most energetic.
Substances ignite under different temperature conditions. Thus, magnesium metal burns only at 2210 °C. For many solids the flame temperature is around 350°C. Matches and kerosene can ignite at 800 °C, while wood can ignite from 850 °C to 950 °C.
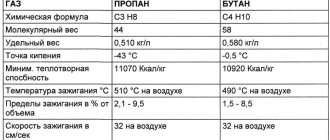
The cigarette burns with a flame whose temperature varies from 690 to 790 °C, and in a propane-butane mixture - from 790 °C to 1960 °C. Gasoline ignites at 1350 °C. The alcohol combustion flame has a temperature of no more than 900 °C.
Meditation on the violet fire
Various visual images are often used to work with consciousness. We ourselves set up the settings for the pictures we want to see. In your personal meditation, fire can change color, burn with different intensities, and even be cold and not scorching.
The image of violet fire is quite contradictory - it is a combination of peace-loving violet energy and the rather aggressive element of fire. The symbolism of a blazing fire is inherently stronger than just the image of light, so it is better to use internal images and pictures with blazing violet fire in cases where you need a particularly powerful boost. But in order to establish initial contact with this energy, you need to try to work with it regularly - at least a few minutes a day. In order for your state to be harmonious, imagine an evenly burning fire and the light calmly emanating from it.
Meditation on the violet flame allows you to fill yourself with spiritual energy, come to terms with any negative event, experience loss and again feel the desire to live. This flame envelops you in pleasant warmth, like a summer wind on a southern night, but does not burn. It gives the ability to forgive and be merciful towards opponents. The creators of violet flame meditation considered it one of the most powerful tools for internal transformation and even called this process “transmutation.” This name implies that a person who has mastered such meditation and placed the violet fire in the heart is radically reborn. You should be accompanied by the feeling that violet energy is capable of melting any negativity into reserves of internal resources. The image may be different. You can imagine that your body is burning with violet fire and is not being burned. Your body is the source of fire.
Another version of the image is violet energy vortexes penetrating your body and emerging from it. Once you are comfortable with passing violet energy through yourself, try to embrace a larger space: your room, home, street, city, country and, ultimately, the whole world. Imagine that you are embracing those around you with violet light, and then your energy spreads further and further - and now you are connected with everyone who lives on this planet. Violet energy allows you to feel the divine presence in all living beings in this world and feel complete unity with them.
Color and its temperature
To imagine what this looks like in real life, consider the color temperature of some sources: xenon car lamps in Figure 3 and fluorescent lamps in Figure 4.
Figure 3 - Color temperature of xenon car lamps.
Figure 4 – Color temperature of fluorescent lamps.
On Wikipedia, I found the numerical values of the color temperatures of common light sources: 800 K - the beginning of the visible dark red glow of hot bodies; 1500-2000 K - candle flame light; 2200 K - incandescent lamp 40 W; 2800 K - 100 W incandescent lamp (vacuum lamp); 3000 K - incandescent lamp 200 W, halogen lamp; 3200-3250 K - typical film lamps; 3400 K - the sun is at the horizon; 4200 K - fluorescent lamp (warm white light); 4300-4500 K - morning sun and lunchtime sun; 4500-5000 K - xenon arc lamp, electric arc; 5000 K - sun at noon; 5500-5600 K - photo flash; 5600-7000 K - fluorescent lamp; 6200 K - close to daylight; 6500 K - standard source of daytime white light, close to midday sunlight; 6500-7500 K - cloudy; 7500 K - daylight, with a large share of scattered light from a clear blue sky; 7500-8500 K - twilight; 9500 K - blue cloudless sky on the north side before sunrise; 10,000 K - "infinite temperature" light source used in reef aquariums (anemone blue tint); 15,000 K - clear blue sky in winter; 20,000 K - blue sky in polar latitudes. Color temperature is a characteristic of the source
Sveta. Any color we see has a color temperature and it doesn’t matter what color it is: red, crimson, yellow, purple, violet, green, white. Works in the field of studying the thermal radiation of a black body belong to the founder of quantum physics, Max Planck. In 1931, at the VIII session of the International Commission on Illumination (CIE, often written in the literature as CIE), the XYZ color model was proposed. This model is a chromaticity diagram. The XYZ model is shown in Figure 5.
Figure 5 – XYZ chromaticity diagram.
The X and Y numeric values define the color coordinates on the chart. The Z coordinate determines the brightness of the color; it is not used in this case, since the diagram is presented in two-dimensional form. But the most interesting thing in this figure is the Planck curve, which characterizes the color temperature of the colors on the diagram. Let's take a closer look at it in Figure 6.
Figure 6 – Planck curve
The Planck curve in this figure is slightly truncated and “slightly” inverted, but this can be ignored. To find out the color temperature of a color, you simply need to extend the perpendicular line to the point of interest (color area). The perpendicular line, in turn, characterizes such a concept as displacement
– degree of color deviation to green or purple. Those who have worked with RAW converters know such a parameter as Tint - this is the offset. Figure 7 displays the color temperature adjustment panel in RAW converters such as Nikon Capture NX and Adobe CameraRAW.
Figure 7 - Panel for setting color temperature for different converters.
It's time to look at how the color temperature is determined not just of an individual color, but of the entire photograph as a whole. Take, for example, a rural landscape on a clear sunny afternoon. Those who have practical experience in photography know that the color temperature at sunny noon is approximately 5500K. But few people know where this figure came from. 5500K is the color temperature of the entire scene
, i.e. the entire image under consideration (picture, surrounding space, surface area). Naturally, an image consists of individual colors, and each color has its own color temperature. What you get: blue sky (12000K), foliage of trees in the shade (6000K), grass in a clearing (2000K), various types of vegetation (3200K - 4200K). As a result, the color temperature of the entire image will be equal to the average value of all these areas, i.e. 5500K. Figure 8 clearly demonstrates this.
Figure 8 – Calculation of the color temperature of a scene shot on a sunny day.
The following example is illustrated in Figure 9.
Figure 9 – Calculation of the color temperature of a scene filmed at sunset.
The picture shows a red flower bud that seems to grow from wheat groats. The picture was taken in the summer at 22:30, when the sun was setting. This image is dominated by a large amount of yellow and orange color tones, although there is a blue tint in the background with a color temperature of approximately 8500K, and there is also an almost pure white color with a color temperature of 5500K. I took just the 5 most basic colors in this image, matched them to a chromaticity chart, and calculated the average color temperature of the entire scene. This is, of course, approximately, but true. There are a total of 272816 colors in this image and each color has its own color temperature. If we calculate the average for all colors manually, then in a couple of months we will be able to get a value that is even more accurate than I calculated. Or you can write a program to calculate and get an answer much faster. Let's move on: Figure 10.
Figure 10 – Calculation of color temperature of other lighting sources
The hosts of the show programs decided not to burden us with color temperature calculations and made only two lighting sources: a spotlight emitting white-green bright light and a spotlight that shines with red light, and the whole thing was diluted with smoke... oh, well, yes - and they installed a presenter bring to Front. The smoke is transparent, so it easily transmits the red light of the spotlight and becomes red itself, and the temperature of our red color, according to the diagram, is 900K. The temperature of the second spotlight is 5700K. The average between them is 3300K. The remaining parts of the image can be ignored - they are almost black, and this color does not even fall on the Planck curve on the diagram, because the visible radiation of hot bodies starts at about 800K (red color). Purely theoretically, one can assume and even calculate the temperature for dark colors, but its value will be negligible compared to the same 5700K. And the last image in Figure 11.
Figure 11 — Calculation of the color temperature of a scene taken in the evening.
The photo was taken on a summer evening after sunset. The color temperature of the sky is located in the region of the blue color tone on the diagram, which, according to the Planck curve, corresponds to a temperature of approximately 17000K. Green coastal vegetation has a color temperature of about 5000K, and sand with algae has a color temperature of about 3200K. The average value of all these temperatures is approximately 8400K.
Burning alcohol lamp
For chemical experiments, small tanks of alcohol are often used. They are called alcohol lamps. The burner wick is soaked with liquid fuel poured through the hole. This is facilitated by capillary pressure. When the free top of the wick is reached, the alcohol begins to evaporate. In the vapor state, it is ignited and burns at a temperature of no more than 900 °C.
The flame of an alcohol lamp has a normal shape, it is almost colorless, with a slight tint of blue. Its zones are not as clearly visible as those of a candle.
Named after the scientist Barthel, the beginning of the fire is located above the burner grid. This deepening of the flame leads to a decrease in the inner dark cone, and the middle section, which is considered the hottest, emerges from the hole.
Characteristic
The flame classification is based on the following characteristics:
- aggregate state of burning compounds. They come in gaseous, airborne, solid and liquid forms;
- type of radiation, which can be colorless, luminous and colored;
- distribution speed. There is fast and slow spread;
- flame height. The structure can be short or long;
- nature of movement of reacting mixtures. There are pulsating, laminar, turbulent movement;
- visual perception. Substances burn with the release of a smoky, colored or transparent flame;
- temperature indicator. The flame can be low temperature, cold and high temperature.
- state of the fuel-oxidizing reagent phase.
Combustion occurs as a result of diffusion or pre-mixing of the active components.
Description of the combustion process
Combustion is a chemical reaction in which large molecules are broken down into smaller, faster molecules by rearranging the bonds between atoms. The reaction releases energy in the form of heat and light. Flame is the visible process of air combustion. Fire appears as a result of an oxidation-reduction reaction. For this reaction to occur, 3 main components must be present:
- fuel: solid, liquid, gaseous (wood, coal, oil, gas and others);
- oxidizing agent: oxygen;
- activator: a trigger to start the process, for example, a spark, the sun, matches, etc.
Wood burning
Classification
Flames are classified according to:
- state of aggregation of flammable substances: flame of gaseous, liquid, solid and aerodispersed reagents;
- radiation: luminous, colored, colorless;
- state of the fuel-oxidizer environment: diffusion, pre-mixed media (see below);
- the nature of the movement of the reaction medium: laminar, turbulent, pulsating;
- temperature: cold, low temperature, high temperature;
- propagation speeds: slow, fast;
- height: short, long;
- visual perception: smoky, transparent, colored.
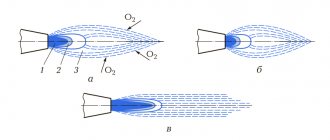
Inside a laminar diffusion flame
3 zones (shells) can be distinguished:
- dark zone (300-350 °C), where combustion does not occur due to a lack of oxidizer;
- luminous zone where thermal decomposition of fuel and its partial combustion occurs (500–800 °C);
- a barely luminous zone, which is characterized by the final combustion of fuel decomposition products and a maximum temperature (900–1500 °C).
The flame temperature depends on the nature of the combustible substance and the intensity of the oxidizer supply.
Flame propagation through pre-mixed medium
(unperturbed), occurs from each point of the flame front normal to the flame surface: the value of such
normal flame propagation velocity
(NFSV) is the main characteristic of the flammable medium. It represents the minimum possible flame speed. NSRP values differ for different combustible mixtures - from 0.03 to 15 m/s.
The spread of flame through real-life gas-air mixtures is always complicated by external disturbing influences caused by gravity, convective flows, friction, and so on. Therefore, the actual flame propagation speeds always differ from normal ones. Depending on the nature of combustion, the flame propagation speeds have the following ranges: for deflagration combustion - up to 100 m/s; during explosive combustion - from 300 to 1000 m/s; during detonation combustion - over 1000 m/s.
The flame of a burning candle has accompanied man for thousands of years.
Oxidizing flame
Located in the upper, hottest part of the flame, where combustible substances are almost completely converted into combustion products. In this area of the flame there is an excess of oxygen and a lack of fuel, so substances placed in this zone are intensively oxidized
.
Restorative flame
This is the part of the flame closest to the center or just below the center of the flame. In this area of the flame there is a lot of fuel and little oxygen for combustion, so if you introduce a substance containing oxygen into this part of the flame, the oxygen is taken away from the substance.
This can be illustrated using the example of the reduction reaction of barium sulfate BaSO4. Using a platinum loop, BaSO4 is taken and heated in the reducing part of the alcohol burner flame. In this case, barium sulfate is reduced and barium sulfide BaS is formed. That's why the flame is called restorative
.
Flame test for sodium.
The color of the flame depends on several factors. The most important are: temperature, the presence of microparticles and ions in the flame, which determine the emission spectrum.
Temperature
The flame temperature depends on the nature of the combustible substance and the intensity of the oxidizer supply. For example:
- The ignition temperature for most solid materials is 300 °C.
- The flame temperature in a burning cigarette is 250-300 °C.
- Match flame temperature 750-1400 °C; while 300 °C is the ignition temperature of wood, and the combustion temperature of wood is approximately 800–1000 °C.
- The combustion temperature of propane-butane is 800-1970 °C.
- The flame temperature of kerosene is 800 °C, in an environment of pure oxygen – 2000 °C.
- The combustion temperature of gasoline is 1300-1400 °C.
- The flame temperature of alcohol does not exceed 900 °C.
- Magnesium combustion temperature – 2200 °C; a significant part of the radiation is in the UV range.
Highest known combustion temperatures:
- dicyanoacetylene C4N2 5260 K (4990 °C) in oxygen and up to 6000 K (5730 °C) in ozone;
- cyanogen (CN)2 4525 °C in oxygen.
Since water has a very high heat capacity, the absence of hydrogen in the fuel eliminates heat loss due to the formation of water and allows the development of high temperatures.
Types of flame
The glow of fire is divided into two types:
- non-luminous;
- glowing.
Almost every glow is visible to the human eye, but not every one is capable of emitting the required amount of luminous flux.
The glow of the flame is determined by the following factors.
- Temperature.
- The density and pressure of the gases that participate in the reaction.
- Presence of solid matter.
The most common cause of glowing is the presence of a solid substance in the flame.
REVIEW OF MICROBURNER TOPEX 44E102
Many gases burn with a faintly luminous or non-luminous flame. Of these, the most common are hydrogen sulfide (blue flame, like combustion), ammonia (pale yellow), methane, carbon monoxide (pale blue flame), and hydrogen. The vapors of some volatile liquids burn with a barely luminous flame (alcohol and carbon disulfide), and the flame of acetone and ether becomes slightly smoky due to the slight release of carbon.
Yellow or orange light
Most likely, the owners of any gas stoves periodically see flames of these colors, but the problem quickly disappears on its own, so the owners do not worry. True, it also happens that the problem becomes permanent, and then the owners may become worried.
In fact, the problem is not so critical, and, most likely, you can even solve it yourself. Most often, it is observed on new stoves purchased no more than a year ago, but this is not an indicator of low quality kitchen appliances - the phenomenon is observed both on cheap Chinese devices and on expensive examples of famous brands. The problem is that any combustion process requires plentiful access of air, and in this case the holes for its suction are clogged, so there is not enough air.
For new stoves, this problem is due to the fact that to prevent oxidation, their parts during storage in a warehouse are covered with a thin film of oil, on which fine dust easily settles. Since the air supply holes are quite small, such dirt can close a significant part of the lumen and cause the characteristic red tint of the flame. During the first year of operation, the problem is usually eliminated - the oil dries out, some of the debris burns out, and if a good owner also regularly cleans the stove, then the problems will quickly disappear.
By the way, the cause of a change in combustion color can also be the displacement of the damper located on the burner. For some manufacturers, its shape is not well thought out, so its fall or partial displacement can partially block the access of air to the combustion site.
Another reason for this phenomenon is that different gases can be used in the systems. Natural gas and propane have different combustion temperatures, and they also need different amounts of air, so when purchasing a stove, it is possible that the equipment is designed for a different type of fuel. You won’t be able to fix anything here - due to incompatibility, the burners will always burn orange.
In most cases, minor blockages are not such a serious problem, but if the phenomenon becomes permanent, the danger may increase. Due to lack of air, a weak flame may simply go out. Most often it goes out in the oven, where it is difficult for air to get in, and you won’t even notice it right away. In this case, the gas, which is turned on but not burning, will begin to accumulate in the room, and in the worst case scenario, it can provoke an explosion that can destroy the entire entrance.
Gas burns orange
| Orange color of burning gas |
The orange color of gas combustion most often occurs if the air suction holes are clogged with dust particles. They create an obstacle to the passage of air. The balance is disrupted when mixing the fuel supply to the burner. Consequently, the gas enters with dust and soot, which is why a different color of the burned gas appears.
Gas equipment is most susceptible to plaque in the first year of use. Because After stamping, the ignition group tube and burner retain an oil film for some time. Therefore, dust sticks and prevents air from passing through, but gas passes through perfectly. There is a large supply of gas to the burner and the balance is upset.
What is fire really?
So fire is not a solid, liquid, gas or plasma. What do we even have left? Probably, we should not consider fire to be matter at all. This is our sensory perception of a chemical reaction called combustion. In a sense, fire is like leaves changing color in autumn, like the smell of ripening fruit, like the flickering light of a firefly. These are all sensory sensations that tell us that some kind of chemical reaction is happening. Fire is different only in that it engages many of our senses at the same time, creating a range of sensations that we expect to see only from something living and material.
Wikipedia defines “what fire is” as follows:
In physics (and in chemistry too), combustion (fire) creates this illusion with the help of fuel, heat and oxygen. When the wood inside a fire reaches combustion temperatures, the walls of its constituent cells disintegrate, releasing sugars and other molecules into the air. They, in turn, react with oxygen in the air, creating water and carbon dioxide. At the same time, the water that is in the tree, evaporating, expands - it breaks the organic matter around it, creating that characteristic crackling sound in a fire, fireplace or stove that we love so much.
As the fire gets hot, the water vapor and carbon dioxide generated during the combustion process dissipate. Losing density, they rise upward in a column. And the expansion, and dispersion, and soaring of gases - all this is caused by the force of gravity, which, in addition to everything, gives the fire a characteristic conical shape. Without gravity, the molecules are not separated by density, and the fire has a completely different shape.
Candle flame
The flame, which every person can observe when a candle, match or lighter burns, is a stream of hot gases that are pulled vertically upward, thanks to the force of Archimedes. The candle wick first heats up and the paraffin begins to evaporate. The lowest part is characterized by a slight blue glow - there is little oxygen and a lot of fuel. It is because of this that the fuel does not burn completely and carbon monoxide is formed, which, when oxidized at the very edge of the flame cone, gives it a blue color.
Due to diffusion, a little more oxygen enters the center. Subsequent oxidation of the fuel occurs there and the temperature increases. But this is not enough for complete combustion of fuel. At the bottom and in the center there are coal particles and unburnt droplets. They glow due to intense heat. But evaporated fuel, as well as combustion products, water and carbon dioxide, practically do not glow. At the very top there is the highest concentration of oxygen. There, the unburned particles that glowed in the center are burning out. It is for this reason that this zone practically does not glow, although the temperature there is the highest.
Read also: Circuit diagram for connecting a triac instead of a relay
Burning alcohol lamp
For chemical experiments, small tanks of alcohol are often used. They are called alcohol lamps. The burner wick is soaked with liquid fuel poured through the hole. This is facilitated by capillary pressure. When the free top of the wick is reached, the alcohol begins to evaporate. In the vapor state, it is ignited and burns at a temperature of no more than 900 °C.
The flame of an alcohol lamp has a normal shape, it is almost colorless, with a slight tint of blue. Its zones are not as clearly visible as those of a candle.
Named after the scientist Barthel, the beginning of the fire is located above the burner grid. This deepening of the flame leads to a decrease in the inner dark cone, and the middle section, which is considered the hottest, emerges from the hole.
What is a flame and why does fire come in different colors?
Flames are presented in the form of hot gases, sometimes containing plasma and solid elements, in which physical and chemical transformations of reagent elements take place, causing glow, heat release, and independent heating.
The gaseous medium of the flame consists of charged ions and radicals, which explains the possibility of electrical conductivity of the flame and its interaction with electromagnetic fields. Using this principle, devices are produced that have the ability, using electromagnetic radiation, to dampen a flame, tear it away from flammable materials, and even change its shape.
Causes of multi-colored flames
Why do we see a bluish fire when we turn on a gas burner and ignite the escaping gas? During combustion, the gas breaks down into oxygen and carbon, releasing carbon monoxide, which causes the blue color.
Why does simple table salt set on fire produce yellow and red colors? The salt contains sodium chloride, which creates yellow-orange flames when burned. Any wooden object or fire made from wood will burn with the same color, since the wood material contains a large amount of similar salts.
Fire also has green shades, why? Their appearance means that burning objects contain phosphorus or copper. Moreover, the copper flame will be bright and blinding, close to white. The cause of a green flame can be the presence of barium, molybdenum, phosphorus, and antimony in combustion objects. The blue color is due to selenium or boron.
Fire without signs of color can only be seen in laboratory conditions. It is possible to understand that something is burning only by the slight vibration of air and the heat generated.
Remember! Fire is very dangerous. Spreads like lightning. Never play with fire. You can only be near a fire in the presence of adults!
Good to know
- All gas appliances pose an increased danger. For this reason, it doesn’t hurt to know some signs of breakdowns and how to fix them. We will identify malfunctions by the color of the flame.
- If your burner produces a yellow or orange flame when operating, this is a sign that there is not enough air mixture. In order for the gas to burn correctly and produce maximum heat, a sufficient amount of air is necessary, which is mixed with the gas in the main burner.
- An imbalance in the fuel-air mixture can occur for various reasons. The air holes are clogged with dust, preventing air flow. Dust accumulations, when burned, create a yellowish or orange flame.
- A yellowish flame is also possible if the gas equipment was purchased incorrectly. When any fuel burns, carbon monoxide is released. Columns that produce a blue flame during operation produce a low level of CO. The presence of an orange or red light indicates the opposite.
- Carbon monoxide poisoning causes flu-like symptoms - headaches, nausea, dizziness. Carbon monoxide is dangerous because its presence often goes unnoticed by people, since it has no color or odor.
Now you know why fire comes in different colors, what determines the color of the flame
Please note: if we see a yellow, red or orange flame on a gas appliance, this can be considered a danger signal. Having discovered this, it is necessary to call qualified specialists who will determine the cause and eliminate the malfunction of the gas equipment
Portal about reinforcement and meshes
Description:
Wetting a copper plate in hydrochloric acid and bringing it to the burner flame, we notice an interesting effect - coloring of the flame. The fire shimmers with beautiful blue-green shades. The spectacle is quite impressive and mesmerizing.
Copper gives the flame a green tint. With a high copper content in the combustible substance, the flame would have a bright green color. Copper oxides give an emerald green color. For example, as can be seen from the video, when copper is wetted with hydrochloric acid, the flame turns blue with a greenish tint. And calcined copper-containing compounds soaked in acid color the flame azure blue.
For reference:
Barium, molybdenum, phosphorus, and antimony also give green color and its shades to fire.
Explanation:
Why is the flame visible? Or what determines its brightness?
Some flames are almost invisible, while others, on the contrary, shine very brightly. For example, hydrogen burns with an almost completely colorless flame; the flame of pure alcohol also shines very weakly, but a candle and a kerosene lamp burn with a bright luminous flame.
The fact is that the greater or lesser brightness of any flame depends on the presence of hot solid particles in it.
Fuel contains carbon in greater or lesser quantities. Carbon particles glow before they burn, which is why the flame of a gas burner, kerosene lamp and candle shines - because it is illuminated by hot carbon particles.
Thus, it is possible to make a non-luminous or weakly luminous flame bright by enriching it with carbon or heating non-combustible substances with it.
How to get multi-colored flames?
To obtain a colored flame, not carbon is added to the burning substance, but metal salts that color the flame in one color or another.
The standard method of coloring a faintly luminous gas flame is to introduce metal compounds into it in the form of highly volatile salts - usually nitrates (salts of nitric acid) or chlorides (salts of hydrochloric acid):
yellow - sodium salts,
red - strontium, calcium salts,
green - cesium salts (or boron, in the form of boronethyl or boronmethyl ether),
blue - copper salts (in the form of chloride).
Selenium colors the flame blue, and boron colors the flame blue-green.
This ability of burning metals and their volatile salts to impart a certain color to a colorless flame is used to produce colored lights (for example, in pyrotechnics).
What determines the color of a flame (in scientific language)
The color of a fire is determined by the temperature of the flame and what chemicals it burns. The high temperature of the flame allows atoms to jump to a higher energy state for some time. When the atoms return to their original state, they emit light at a specific wavelength. It corresponds to the structure of the electronic shells of a given element.
The children and I wondered: why is the fire from the fire and the candle yellow, but the fire from the gas on the stove is blue?
And we learned a lot of interesting things! It turns out that the color of the flame depends on the temperature of the fire and the chemical composition of the substances that burn in it. Most often, quite exotic ingredients are required to color the flame in an unusual color. But we also have substances at home that will burn in different colors. So with the help of them we decided to paint the fire.
After reading various theories on the Internet, we found the following recipes:
1. Yellow
the flame colors sodium (available to us in the form of sodium chloride - common table salt and sodium bicarbonate - baking soda).
2. Blue
Methane gas (the one we have in our gas stove) and alcohol are burning.
3. Green color
gives the flame copper (ordinary copper wire or, for example, copper sulfate powder)
With other colors it is more difficult, the components in them are very rare. Well, what if you have them at home or you know where you can get them?
4. Green color
and besides copper, barium, molybdenum, phosphorus, and antimony also give its shades to fire.
5. Blue color
and its shades give copper chloride, selenium, boron
6. Red color
give strontium and calcium salts and lithium chloride.
7. Crimson color
- potassium chloride.
But in our home, of all the above, we found only methane, sodium and copper. That's why we experimented with them.
The easiest way to get a blue flame is to turn on the gas burner and a blue light lights up on the stove :)
If you insert an ordinary copper wire bent into a ring at the end and wait a few seconds until the wire gets hot, you can see a green flame.
True, the wire very quickly becomes covered with oxide, and the copper stops burning. Therefore, it is necessary to either clean the wire often or use copper sulfate. To do this, we simply dipped the tip of the wire moistened with water into blue copper sulfate powder and brought it to the fire. The green color of the flame lasts much longer, although even here the crystals oxidize very quickly.
And if you dip the same wire, moistened with water, in salt or soda and bring it into the blue flame of a gas burner, the flame will immediately turn yellow-orange. The way we are used to seeing him in the fire. After all, the fire's orange color is also due to sodium - wood contains a lot of salts.
For many centuries, fire has played a very important role in human life. Without it it is almost impossible to imagine our existence. It is used in all areas of industry, as well as for cooking, warming the home and promoting technological progress.
Fire first appeared in the Early Paleolithic era. Initially, it was used in the fight against various insects and attacks by wild animals, and also provided light and warmth. And only then the flames of fire were used in cooking, making dishes and tools. This is how fire entered our lives and became man’s “indispensable assistant.”
Many of us have noticed that flames can vary in color, but not many know why the fire element has a variegated color. Typically, the color of a fire depends on what chemical is being burned in it. Due to the exposure to high temperature, all the atoms of the chemicals are released, thus giving the hue to the fire. A large number of experiments were also carried out, which will be written about in this article below, in order to understand how these substances affect the color of the flame.
Since ancient times, scientists have made efforts to understand what chemicals burn in a flame, depending on what color the fire takes.
We can all see a light with a blue tint when cooking at home. This is predetermined by highly combustible carbon and carbon monoxide, which gives the light its blue tint. Sodium salts, which are endowed with wood, give the fire a yellow-orange hue, which burns with an ordinary fire or matches. If you sprinkle the stove burner with regular salt, you can get the same color. Copper gives fire its green color. With a very high concentration of copper, the light has a very bright shade of green, which is virtually identical to colorless white. This can be observed if you sprinkle copper shavings on the burner.
Experiments were also carried out with an ordinary gas burner and various minerals in order to establish their constituent chemical substances. To do this, carefully take the mineral with tweezers and bring it to the fire. And, based on the shade that the fire took, one can draw conclusions about the various chemical additives that are present in the element. Minerals such as copper, barium, phosphorus, molybdenum give a green tint, while boron and antimony give a blue-green color. Selenium also gives the flame its blue color. A red flame is obtained by adding lithium, strontium and calcium, a purple flame is obtained by the combustion of potassium, and a yellow-orange color is produced by sodium.
To study various minerals and determine their composition, a Bunsen burner is used, invented in the 19th century by Bunsen, which produces a colorless flame that does not interfere with the course of the experiment.
It was Bunsen who became the founder of the method for determining the chemical composition of a substance based on the color palette of the flame. Of course, before him there were attempts to conduct such experiments, but such experiments were not successful, since there was no burner. He introduced various chemical components into the fiery element of the burner on a wire made of platinum, because platinum does not affect the color of the fire in any way and does not give it any shade.
At first glance, it may seem that there is no need for any complex chemical research; bring the component to the fire - and you can instantly see its composition. However, not all so simple. In nature, substances in their pure form are very rare. As a rule, they include a considerable range of different impurities that can change color.
Consequently, using the characteristic properties of molecules and atoms to emit light of a certain color range, a method was created for determining the chemical composition of substances. This method of determination is called spectral analysis. Scientists are studying the spectrum that the substance emits. For example, during combustion, it is compared with the spectra of known components, and thus its chemical composition is established.
A very beautiful scientific experiment from Professor Nicolas, “Colored Flames,” allows you to create flames of four different colors using the laws of chemistry.
The set is most interesting, we really saw enough of the flames, an amazing sight! It’s interesting for everyone: both adults and children, so I highly recommend it! The advantage is that this experiment with fire can be done at home, you don’t have to go outside. The set includes bowls in which a tablet of dry fuel burns, everything is safe, and can be placed on a wooden floor (or table).
It is better, of course, to conduct the experiment under adult supervision. Even if the children are already quite big. Fire is still a dangerous thing, but at the same time... creepy (this is the word that fits here very accurately!) interesting!! :-))
See photos of the set packaging in the gallery at the end of the article.
The Colored Flame kit contains everything you need to carry out the experiment. The set includes:
- potassium iodide,
- calcium chloride,
- hydrochloric acid solution 10%,
- copper sulfate,
- nichrome wire,
- copper wire,
- sodium chloride,
- dry fuel, evaporation cup.
The only thing I have some complaints about the manufacturer is that I expected to find a mini-brochure in the box describing the chemical process that we are seeing here and an explanation of why the flame becomes colored. There was no such description here, so you’ll have to turn to the chemistry encyclopedia (). If, of course, there is such a desire. And older children, of course, have a desire! Younger children, of course, do not need any explanations: they are simply very interested in watching how the color of the flame changes.
On the back of the packaging box it is written what needs to be done to make the flame become colored. At first they did it according to the instructions, and then they just started sprinkling the flames with different powders from jars (when they were sure that everything was safe) :-)) - the effect was amazing. Flashes of red flame in yellow, bright light green flame, green, purple... the sight is simply mesmerizing.
It’s very cool to buy for some holiday, it’s much more interesting than any firecracker. And it will be very great for the New Year. We burned during the day; it would have been even more spectacular in the dark.
We still have the reagents left after burning one tablet, so if we take another tablet (purchase separately), we can repeat the experiment. The clay cup washed quite well, so it will be enough for many experiments. And if you are at the dacha, then you can sprinkle the powder on the fire in the fire - then, of course, it will quickly end, but the spectacle will be fantastic!
I am adding brief information about the reagents that come with the experiment. For inquisitive kids who are interested in learning more.
Flame coloring
In laboratory conditions, it is possible to achieve a completely colorless fire, which can only be determined by the vibration of the air in the combustion area. Household fire is always “colored”.
The color of a fire is determined by the temperature of the flame and what chemicals it burns. The high temperature of the flame allows atoms to jump to a higher energy state for some time. When the atoms return to their original state, they emit light at a specific wavelength. It corresponds to the structure of the electronic shells of a given element.
The
blue
light, for example, that can be seen when natural gas burns, is caused by carbon monoxide, which gives the flame its hue. Carbon monoxide, a molecule made up of one oxygen atom and one carbon atom, is a byproduct of the combustion of natural gas.
Potassium - violet flame
Potassium is the most electropositive metal after rubidium and cesium. In clean dry air at ordinary temperature it does not change, in ordinary air it is covered with a layer of caustic potassium and carbon dioxide of its salt; in a fresh cut it glows in the dark, but in thin plates it oxidizes so quickly that it can catch fire; molten and heated, it also burns; its flame is violet in color.
Due to this tendency to oxidize, it is necessary to store it under oil.
When soluble calcium salts are added to the flame, the flame turns brick-red.
.
1) In green
The color
of the flame
is colored by boric
acid
or copper (brass) wire dipped in
hydrochloric acid
.
The flame turns red
colors chalk soaked in the same
hydrochloric acid
.
When strongly calcined in thin fragments, Ba-containing (Barium-containing) minerals color the flame yellow-green. The coloring of the flame can be enhanced if, after preliminary calcination, the mineral is moistened in strong hydrochloric acid.
Barium, molybdenum, phosphorus, and antimony also give green color and its shades to fire.
Copper nitrate and hydrochloric acid solutions are blue or green; When ammonia is added, the color of the solution changes to dark blue.
Yellow flame - salt
A yellow
flame
requires the addition of table
salt
, sodium nitrate or sodium chromate.
Try sprinkling a little table salt on the burner of a gas stove with a transparent blue flame - yellow tongues will appear in the flame. Such a yellow-orange flame
give sodium salts (and table salt, remember, is sodium chloride).
Yellow is the color of sodium in the flame. Sodium is present in any natural organic material, which is why we usually see yellow flames. And yellow color can drown out other colors - this is a feature of human vision.
Yellow flames appear when sodium salts decompose. Wood is very rich in such salts, so an ordinary forest fire or household matches burn with a yellow flame.
Why should you contact PlitHome?
We are a company with a narrow specialization, which guarantees our competence in troubleshooting problems of any complexity
We do not repair other types of appliances, which allows us to focus our attention on stoves, ovens and hobs. We work throughout Moscow and St. Petersburg. We offer our clients a free visit from a technician to their home.
Thanks to this, you will not have to waste time, money and nerves on delivering devices to our office. We use professional tools and original spare parts. Moreover, the latter are always available in our own warehouse. We regularly replenish our assortment based on the release of new models to the market. We carry out all repair work in the presence of the client. You can personally verify the presence of any damage. At the same time, you will be confident in the transparency of our pricing policy. Official cooperation. We issue a warranty card indicating the type of work and the article numbers of the installed spare parts. You can trust us.
If a yellow or red flame forms on the burners during combustion, then call us or request a call back. The master will arrive urgently or at a time convenient for you. We work with both domestic and imported models of equipment, including Gorenje, Indesit, Bosch, Mechta, Hansa, Gefest and others.
How is fire temperature measured?
It is impossible to measure the temperature of a fire without special equipment; household appliances are not suitable for this. To do this, you need to use a professional pyrometer.
This device works contactless. All you need to do is simply point it at an open fire, and the device will determine the approximate temperature of the fire using infrared radiation. Measurement can be carried out from any distance, but the device must see the object being measured, the air must be transparent (i.e., not smoke-filled).
Typically, a pyrometer shows the temperature of the fire from a regular fire or fireplace in the range of 750 to 1200 degrees Celsius.
Fire temperature measurement
Lights in liquid
Pour concentrated sulfuric acid into a small glass cylinder to a height of 10-12 cm
Carefully pour ethyl alcohol C2H5OH on top, filling the cylinder almost to the top. Then pour pre-crushed potassium permanganate crystals into the cylinder
Falling grains of KMnO4 reach the boundary separating alcohol and sulfuric acid and cause flashes of fire, so that within a few minutes you can observe real fireworks in the liquid!
Fires in liquids occur when ethanol instantly ignites upon contact with manganese(VII) oxide, which is formed by the reaction of KMnO4 and H2SO4.
Flame color and chemical composition
The color of the flame may vary depending on the chemical impurities contained in the logs or other flammable substance. The flame may contain, for example, sodium impurities.
Even in ancient times, scientists and alchemists tried to understand what kind of substances burned in fire, depending on the color of the fire.
- Sodium is a component of table salt. When sodium is heated, it turns bright yellow.
- Calcium may be released into the fire. We all know that milk contains a lot of calcium. It's metal. Hot calcium turns bright red.
- If phosphorus burns in a fire, the flame will turn greenish. All these elements are either contained in wood or enter the fire with other substances.
- Almost everyone at home has gas stoves or water heaters, the flames of which are colored blue. This is due to combustible carbon, carbon monoxide, which gives this shade.
Mixing the colors of a flame, like mixing the colors of a rainbow, can produce white, which is why white areas are visible in the flames of a fire or fireplace.
Flame temperature when burning certain substances:
What determines the color of the flame?
Flames come in different colors. Look into the fireplace. Yellow, orange, red, white and blue flames dance on the logs. Its color depends on the combustion temperature and the combustible material. To visualize this, imagine the spiral of an electric stove. If the tile is turned off, the spiral turns are cold and black. Let's say you decide to heat up the soup and turn on the stove. At first the spiral turns dark red. The higher the temperature rises, the brighter the red color of the spiral. When the tile reaches its maximum temperature, the coil turns orange-red.
Naturally, the spiral does not burn. You don't see the flame. She's just really hot. If you heat it further, the color will change. First, the color of the spiral will turn yellow, then white, and when it heats up even more, a blue glow will emanate from it.
Something similar happens with fire. Let's take a candle as an example. Different areas of a candle flame have different temperatures. Fire needs oxygen. If you cover a candle with a glass jar, the fire will go out. The central area of the candle flame adjacent to the wick consumes little oxygen and appears dark. The top and side areas of the flame receive more oxygen, so these areas are brighter. As the flame moves through the wick, the wax melts and crackles, breaking into tiny carbon particles. (Coal also consists of carbon.) These particles are carried upward by the flame and burn. They are very hot and glow like the spiral of your tile. But the carbon particles are much hotter than the coil of the hottest tile (carbon combustion temperature is approximately 1,400 degrees Celsius). Therefore, their glow is yellow. Near the burning wick, the flame is even hotter and glows blue.
The flames of a fireplace or fire are mostly motley in appearance. Wood burns at a lower temperature than a candle wick, so the base color of the fire is orange rather than yellow. Some carbon particles in a fire flame have a fairly high temperature. There are few of them, but they add a yellowish tint to the flame. Cooled particles of hot carbon are soot that settles on the chimneys. The burning temperature of wood is lower than the burning temperature of a candle. Calcium, sodium and copper, when heated to high temperatures, glow in different colors. They are added to rocket powder to color the lights of holiday fireworks.
Flame color and chemical composition
The color of the flame may vary depending on the chemical impurities contained in the logs or other flammable substance. The flame may contain, for example, sodium impurities.
Even in ancient times, scientists and alchemists tried to understand what kind of substances burned in fire, depending on the color of the fire.
- Sodium is a component of table salt. When sodium is heated, it turns bright yellow.
- Calcium may be released into the fire. We all know that milk contains a lot of calcium. It's metal. Hot calcium turns bright red.
- If phosphorus burns in a fire, the flame will turn greenish. All these elements are either contained in wood or enter the fire with other substances.
- Almost everyone at home has gas stoves or water heaters, the flames of which are colored blue. This is due to combustible carbon, carbon monoxide, which gives this shade.
Mixing the colors of a flame, like mixing the colors of a rainbow, can produce white, which is why white areas are visible in the flames of a fire or fireplace.
Flame temperature when burning certain substances:
What determines the color of the flame
Something similar happens with fire. Let's take a candle as an example. Different areas of a candle flame have different temperatures. Fire needs oxygen. If you cover a candle with a glass jar, the fire will go out. The central area of the candle flame adjacent to the wick consumes little oxygen and appears dark. The top and side areas of the flame receive more oxygen, so these areas are brighter.
As the flame moves through the wick, the wax melts and crackles, breaking into tiny carbon particles. (Coal also consists of carbon.) These particles are carried upward by the flame and burn. They are very hot and glow like the spiral of your tile. But the carbon particles are much hotter than the coil of the hottest tile (carbon combustion temperature is approximately 1,400 degrees Celsius). Therefore, their glow is yellow. Near the burning wick, the flame is even hotter and glows blue.
The flames of a fireplace or fire are mostly motley in appearance. Wood burns at a lower temperature than a candle wick, so the base color of the fire is orange rather than yellow. Some carbon particles in a fire flame have a fairly high temperature. There are few of them, but they add a yellowish tint to the flame. Cooled particles of hot carbon are soot that settles on the chimneys. The burning temperature of wood is lower than the burning temperature of a candle. Calcium, sodium and copper, when heated to high temperatures, glow in different colors. They are added to rocket powder to color the lights of holiday fireworks.
Candle and magic wand
You can light a candle without matches in a completely harmless way, but under the supervision of a teacher or the leader of a chemistry club. To do this, you will first need to imitate a real candle - pour melted paraffin or stearin on the outside of a thin glass test tube (you can even just drip paraffin from a burning candle, holding it at an angle). Ethanol C2H5OH (ethyl alcohol) is poured into a test tube to fill 1/2 of its volume and a metal cap with a hole is put on it, through which a wick of 5 to 10 threads of paper yarn is passed. The cap must also be doused with molten paraffin.
How to measure the temperature of coals in a barbecue
The temperature of the coals in the grill can be determined with a certain accuracy without the use of special instruments. All you need to do is place your hand about 10 cm above the grill.
The number of seconds you can withstand will be equal to the approximate temperature of the coals:
- 1 sec – from 350 °C and above
- 2 sec. – around 300 °C
- 3 sec. – about 250 °C
- 4 sec. – 220 °C
- 5 sec. – less than 200 °C
Temperature of coals in the grill
conclusions
In principle, most of the problems that arise during the operation of the stove and which lead to the impossibility of igniting the burners do not pose a danger to humans
However, keep in mind that we are still talking about, so caution never hurts
If you find that you can make repairs yourself, clean the mechanism, check the operation - do it, but if in your case it comes to replacing elements inside the device, then it is better to refrain. Unprofessional repairs may result in gas leaks or electric shock.
It doesn’t matter where you smelled domestic gas, in your country house, in an apartment or in a stairwell, a gas leak is a serious danger to the property and health of the people around you. In the event of a prolonged leak, gas accumulates in a confined space, turning into an explosive mixture that can detonate at any moment
That is why we recommend calling the gas service at the slightest smell of gas. But if you live in a country house far from gas facilities and calling a gas technician to your home is impossible for some reason, then read our article to the end and you will learn how to find a gas leak in the stove and quickly eliminate it.