4.2
Average rating: 4.2
Total ratings received: 261.
4.2
Average rating: 4.2
Total ratings received: 261.
The physical properties of metals distinguish them from non-metals. All metals, except mercury, are solid crystalline substances that are reducing agents in redox reactions.
Position in the periodic table
Metals occupy groups I-II and secondary subgroups of groups III-VIII. Metallic properties, i.e. the ability to donate valence electrons or be oxidized increases from top to bottom as the number of energy levels increases. From left to right, metallic properties weaken, so the most active metals are in groups I-II, the main subgroups. These are alkali and alkaline earth metals.
The degree of activity of metals can be determined by the electrochemical series of voltages. Metals that come before hydrogen are the most active. After hydrogen come weakly active metals that do not react with most substances.
Rice. 1. Electrochemical series of metal voltages.
Porosity
Among the physical, technological and mechanical properties of materials, porosity is not the least important. This is the degree to which the volume of the product is filled with pores.
In this context, pores are tiny cells filled with water or air. They can be large and small, open and closed. If small pores, for example, are filled with air, this increases the thermal insulation properties of the material. The amount of porosity helps to judge other important characteristics - durability, strength, water absorption, density.
Open pores communicate both with the environment and with each other, and can be artificially filled with water when the material is immersed in liquid. Usually alternate with closed ones. In sound-absorbing materials, for example, open porosity and perforation are artificially created for more intense absorption of sound energy.
Closed pores are characterized by distribution and size as follows:
- Integral curve of distribution of pore volume per unit volume over their radii.
- Differential distribution curve for pore volume radii.
Structure
Regardless of activity, all metals have a common structure. Atoms in a simple metal are not arranged chaotically, as in amorphous substances, but in an orderly manner - in the form of a crystal lattice. A metal bond holds the atoms in one position.
This type of connection is carried out due to positively charged ions located at the nodes of a crystal cell (lattice unit), and negatively charged free electrons, which form the so-called electron gas. Electrons separated from atoms, turning them into ions, and began to move randomly in the lattice, holding the ions together. Without electrons, the lattice would disintegrate due to the rejection of equally charged ions.
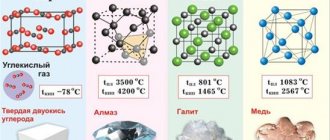
There are three types of crystal lattice. Body-centered cubic consists of 9 ions and is characteristic of chromium, iron, and tungsten. Face-centered cubic contains 14 ions and is characteristic of lead, aluminum, and silver. A hexagonal close-packed lattice of zinc, titanium, and magnesium consists of 17 ions.
Rice. 2. Types of crystal lattices.
Density
One of the most important properties in materials science. Density is divided into three categories:
- True. The mass per unit volume of a material considered to be absolutely dense.
- Average. This is already the mass of a unit volume in the natural state of the material (with pores and voids). Thus, the average density of products made from the same material can be different, depending on the hollowness and porosity.
- Bulk. Used for bulk materials - sand, crushed stone, cement. This is the name given to the ratio of the mass of powdery and granular materials to the entire volume they occupy (the space between particles is also included in the calculations).
You may be interested in: The Throne of Ivan the Terrible: description, where it came from, legends associated with it
The density of the material affects its technological characteristics - strength, thermal conductivity. It will directly depend on porosity and humidity. With increasing humidity, the density will correspondingly increase. This is also a characteristic indicator for determining the cost-effectiveness of a material.
Properties
The structure of the crystal lattice determines the basic physical and chemical properties of metals. Metals shine, melt, and conduct heat and electricity. Industry and metallurgy have found application for the physical properties of metals in the manufacture of parts, foil, machine bodies, mirrors, household and industrial chemicals. The characteristics of metals and their uses are presented in the table of physical properties of metals.
| Properties | Peculiarities | Examples | Application |
| Metallic shine | Ability to reflect sunlight | The most shiny metals are Hg, Ag, Pd | Making mirrors |
| Density | Light - have a density of less than 5 g/cm3 | Na, K, Ba, Mg, Al. The lightest metal is lithium with a density of 0.533 g/cm3 | Manufacturing of cladding and aircraft parts |
| Heavy - have a density greater than 5 g/cm3 | Sn, Fe, Zn, Au, Pb, Hg. The heaviest is osmium with a density of 22.5 g/cm3 | Use in alloys | |
| Plastic | Ability to change shape without destruction (can be rolled into thin foil) | The most ductile are Au, Cu, Ag. Brittle – Zn, Sn, Bi, Mn | Forming, pipe bending, wire making |
| Hardness | Soft - cut with a knife | Na, K, In | Making soap, glass, fertilizers |
| Hard – comparable in hardness to diamond | The hardest is chrome, it cuts glass. | Manufacturing of load-bearing structures | |
| Melting temperature | Low-melting - melting point below 1000°C | Hg (38.9°C), Ga (29.78°C), Cs (28.5°C), Zn (419.5°C) | Production of radio equipment, tinplate |
| Refractory – melting point above 1000°C | Cr (1890°С), Mo (2620°С), V (1900°С). The most refractory is tungsten (3420°C) | Manufacturing of incandescent lamps | |
| Thermal conductivity | Ability to transfer heat to other bodies | Ag, Cu, Au, Al conduct current and heat best | Cooking in metal containers |
| Electrical conductivity | The ability to conduct electric current due to free electrons | Transmission of electricity through wires |
Rice. 3. Examples of the use of metals.
What physical properties of metals are used in technology?
The technical side of the question regarding metals has a clear answer - absolutely all physical properties are used. Only the degree of influence of certain properties changes. In one direction the emphasis is on density, and in the other the melting point. Next, I will dwell in detail on each of the properties in the physics of metals.
1) Density
A basic physical quantity that is important in 95% of technical issues of use. Turning to terminology, the density of a substance is the ratio of mass to the volume of a metal body. A physical property is expressed in terms of grams divided by cubic centimeters. Less commonly used are kilograms per cubic meters.
The picture above, taken from technical literature, makes it possible to find out the density of most popular grades of steel, cast iron and other ferrous or non-ferrous alloys. To measure the density of non-standard alloys that are not indicated in the template tables, in 95%+ of cases the hydrostatic method is used. The remaining 5% use the pycnometric method.
GOST standards for the hydrostatic method of measuring density:
- 20018-74;
- 25281-82;
- 15239-69;
- TU 48-19-76-90.
The measurements are based on well-wetting materials that do not react with metal + do not volatilize during the measurements themselves. Usually the simplest option is used - distilled water.
Important: the value of density is decisive in the manufacture of parts in aircraft and rocket technology. The resulting structures simply must combine strength and lightness.
The issue of optimizing weight and strength is one of the main problems of modern design. It is the density in this matter that is of decisive importance, and therefore this fact puts the physical parameter of metals in the top 3 in importance from the entire attached list of properties of a group of elements.
2) Melting point
Most metals have a number of original properties that are unique to them. Each has its own critical point, at which the crystal lattice is destroyed and the transition from solid to liquid occurs while maintaining the volume of the metal element. The described process is called metal smelting and in the metallurgical industry it is the basis of production.
Important: technology uses alloys made of pure metals and alloying additives. It is impossible to obtain the desired properties without using the melting process.
New compounds are formed by mixing the crystal lattices of pure elements. The melting point is a variable value, depending on the concentration of the components included in the alloy.
Depending on the melting point, metals are divided into 3 categories - low-melting, medium-melting and refractory. The former have an upper melting threshold of less than 1,000 Celsius, and the latter more than 1,500 degrees.
The use of refractory and low-melting metals in technology is discussed below.
12 physical and chem. characteristics of the metal that melts in the hands
| Refractory metals | Low-melting metals |
| Application in welding. We all know about tungsten alloy electrodes. In this case, the metal acts as the basis for the consumable. | Liquid metal thermal media have found application in the energy industry and mechanical engineering. |
| Elements in electronics. | Manufacturing of lost wax models. |
| Space and aviation. Some alloys are used in supersonic aviation and spacecraft production. | Vacuum technology. Application in seals, soldering seams and the like. |
| Military industry. As a rule, structurally important elements that must be protected from high temperatures and melting are packaged in shells made of refractory metal. | Microelectronics, namely the coating of various sensors, fuses and, of course, use as solders. |
| They are used in the development of vacuum-type equipment. | Used as a base for hot melt lubricant for metals. |
The most popular and obvious use of refractory metals is filaments in lamps. Rolled metal products include drawing strips, foil, pipes and wire.
3) Electrical conductivity
This property is based on the metal’s ability to conduct electric current. The value is the reciprocal of the electrical resistance. The designation of the parameter in the technical literature is “G”, and the unit of measurement in accordance with the international system is Siemens (Sm).
Silver boasts the highest conductivity of electric current (62,500,000 S/m). Since the metal itself belongs to the “noble” group, making wiring from it is very expensive. Copper (59,500,000 S/m) is used as a cheaper alternative. Its higher melting point makes it possible to extend the life of a structural element whose purpose is to conduct electricity.
Please note: any of the alloys has much lower electrical conductivity than the pure substance.
The reason for this is the merging of the structural network of elements, which causes the normal operation of electrons inside the new metallic substance to cease. The formation of a knowledge base around the property under consideration occurred through the theory of electrical conductivity of metals.
It includes 6 points:
- High conductivity is related to the number of free electrons;
- The generation of current occurs due to external influence on the metal, as a result of which the movement of electrons inside the element is ordered.
- The strength of the current passing through the metal is calculated based on Ohm's law.
- A different number of elementary particles affects the resistance value.
- Current in the circuit occurs immediately after exposure to electrons.
- As the temperature increases, the resistance of the metal also increases.
Metals from the alkaline group boast the highest electrical conductivity, but due to their limitations in other properties (melting point and chemical reactivity), their use in technology and industry is extremely limited.
Where are electrically conductive metals used:
- when grounding electrical installations;
- for the purpose of equalizing potentials;
- like lightning rods.
Well, the main function of conductors is to deliver electricity. Science bypassing this property would not allow the development of technical progress as such in principle.
6 steps on how to distinguish copper from brass at home
4) What other physical properties of metals are used in technology: thermal conductivity
Thermal conductivity of substances is an integral part of thermodynamics. In relation to metal, this property shows how well the material is able to distribute heat over the entire plane of the metal object. The transport of thermal energy occurs due to the movement of elementary particles inside the element - atoms, electrons, and so on.
Reference values of thermal conductivity for popular metals and alloys are presented in the picture above. More detailed tables are presented in specialized literature on materials science.
Please note: thermal conductivity values are given in the range from 0 to 600 Celsius.
It is impossible to say about the total advantage of metals with high or low thermal conductivity. It all depends on the scope of application of the material.
In what areas is this parameter important:
- construction. Priority on low conductivity materials. In such rooms the temperature will remain optimal both in summer and winter;
- heating systems. Relevant in the production of radiators and pipes for heat transportation;
- technique. In certain areas of instrumentation, protection against overheating is important. With such requirements, the choice of shell material is based on the thermal conductivity of the material.
It is important to understand that when new types of alloys are formed, the heat conductivity parameter changes. To find out the current values, experienced determination methods are used. The private choice depends on the characteristics of the metal being studied.
Basic physical properties of metals:
What have we learned?
From the 9th grade lesson we learned about the physical properties of metals. We briefly examined the position of metals in the periodic table and the structural features of the crystal lattice. Due to their structure, metals have plasticity, hardness, the ability to melt, and conduct electric current and heat. The properties of metals are heterogeneous. There are light and heavy metals, fusible and refractory, soft and hard. Physical properties are used to make alloys, electrical wires, dishes, soap, glass, and structures of various shapes.
Definition
Physical properties of a material are all properties that are inherent in substances without chemical influence on them.
Any material remains unchanged (by itself) under one condition - as long as its composition, as well as the structure of its molecules, remains unchanged. If the substance is non-molecular, its composition and connections between atoms remain the same. And differences in the physical properties and other characteristics of the material help to separate mixtures consisting of it.
You may be interested in: Is idle curiosity safe?
It is also important to know that the physical properties of a material may be different for its different aggregate materials. For example, the thermal, electrical, mechanical, physical, optical properties of a substance depend on the chosen direction in the crystal.
Moisture release
This is the ability of a material to release moisture into the environment. When exposed to air, raw materials and products retain their moisture only under certain conditions - at relative equilibrium air humidity. If the indicator is below this value, then the material begins to release moisture into the atmosphere and dry out.
The speed of this process depends on several factors: on the difference between the humidity of the material itself and air humidity (the higher it is, the more intense the drying), on the properties of the material itself - its porosity, nature, hydrophobicity. Thus, a raw material with large pores, hydrophobic, will release liquid more easily than a hydrophilic material with small pores.
Water permeability
Water permeability is the ability of a material to give off liquid when dried and absorb water when wet.
When studying the physical properties of materials, you need to pay attention to the fact that saturation with water can occur in two ways: when exposed to a substance in a liquid state or when exposed to only its vapor.
This leads to two other important properties: hygroscopicity and water absorption.
Air resistance
Air resistance is the ability of a material to withstand repeated systematic drying and moistening for a long time without loss of its mechanical density, as well as without significant deformation.
Some materials begin to swell when periodically moistened, some shrink, and some warp too much. Wood, for example, is subject to alternating deformations. When cement is frequently moistened and dried, it tends to deteriorate and crumble.
Hygroscopicity
How is this physical property of materials determined in materials science? Hygroscopicity is the ability to absorb water vapor and retain it inside itself as a result of capillary condensation. Directly depends on the relative humidity and air temperature, the size, type and number of pores of the substance, its nature.
If a material actively attracts water molecules with its surface, then it is called hydrophilic. If the material, on the contrary, repels them from itself, then it is called hydrophobic. In addition, some hydrophilic materials are highly soluble in water, while hydrophobic materials are resistant to aqueous media.