There are many different chemical compounds known in the world: about hundreds of millions. And all of them, like people, are individual. It is impossible to find two substances that have the same chemical and physical properties with different compositions.
One of the most interesting inorganic substances existing in the world are carbides. In this article we will discuss their structure, physical and chemical properties, applications and analyze the intricacies of their production. But first, a little about the history of the discovery.
Story
Metal carbides, the formulas of which we give below, are not natural compounds. This is due to the fact that their molecules tend to disintegrate when interacting with water. Therefore, it is worth talking here about the first attempts to synthesize carbides.
Since 1849, there have been references to the synthesis of silicon carbide, but some of these attempts remain unrecognized. Large-scale production began in 1893 by the American chemist Edward Acheson using a method that was later named after him.
The history of the synthesis of calcium carbide is also not very rich in information. In 1862, the German chemist Friedrich Wöhler obtained it by heating fused zinc and calcium with coal.
Now let's move on to more interesting sections: chemical and physical properties. After all, they contain the whole essence of the use of this class of substances.
A plant for the production of calcium carbide will be launched in Inta in 2022
In Inta, it is planned to launch a plant for the production of calcium carbide in 2022. The head of the Inta city district, the head of the administration, Vladimir Kiselev, told Komiinform about this.
“The organization registered with Inta, rented premises and land, and began making monthly contributions to the district budget. Production is planned to be launched in 2022, initially we are talking about creating 70 jobs,” noted V. Kiselev.
Let us remind you that the calcium carbide production plant was part of a comprehensive project for the development of the single-industry town Inta, which a team of Inta managers defended after training under the presidential priority program “Comprehensive Development of Single-Industry Towns.”
Calcium carbide production was the least expensive project. Calcium carbide is a chemical compound of calcium with carbon necessary for the production of acetylene, artificial rubber or various mineral fertilizers. Calcium carbide is obtained by fusing coke and quicklime in electric furnaces. Molten carbide is released from the furnace into special molds - molds, in which it hardens. The solidified calcium carbide is crushed and sorted into pieces of certain sizes. Raw materials for the production of calcium carbide in the vicinity of Inta are available in unlimited quantities.
The creation of such production required relatively small investments - about 200 million rubles. Construction of a modular plant takes only nine months. Profit from sales of products has a high added value.
V. Kiselev also recalled that one of the achievements of 2022 was the inclusion of Inta in the Arctic zone by decree of the Russian President.
“Prospective directions for the development of the Arctic region are being discussed at the highest level,” he continued. — The Russian Arctic today is becoming a special economic zone. Any entrepreneur who is ready to invest at least 1 million rubles in the Arctic zone will be able to obtain resident status, which opens access to tax benefits and non-tax preferences. And we are sure that there will definitely be people who want to build their business in the north. The head of the region, Vladimir Uyba, speaks about this at all levels: “The development potential of Vorkuta and Inta has not been exhausted.”
Continuing the topic of economic development of the city, V. Kiselev also said that after the visit of the head of the region, Vladimir Uyb, a positive trend has emerged at the Inta Pripolarnaya Agrocomplex: all arrears on taxes and utility bills have been closed, wages are paid on time. A new development plan is being drawn up, which includes the construction of a new farm for cattle, the resumption of egg production, the modernization of the reindeer herding complex - deeper processing of venison meat, and the renewal of existing agricultural equipment with modern ones.
It is also planned to build and commission a gas boiler house of PJSC T Plus. The work period is 2021-2023. During construction, additional jobs will appear, and after the facility is put into operation, the reliability of the city’s heat supply will be increased and the environmental situation will be improved.
Physical properties
Absolutely all carbides are distinguished by their hardness. For example, one of the hardest substances on the Mohs scale is tungsten carbide (9 out of 10 possible points). In addition, these substances are very refractory: the melting point of some of them reaches two thousand degrees.
Most carbides are chemically inert and react with small amounts of substances. They are not soluble in any solvents. However, dissolution can be considered interaction with water with the destruction of bonds and the formation of metal hydroxide and hydrocarbon.
We will talk about the last reaction and many other interesting chemical transformations involving carbides in the next section.
Chemical properties
Almost all carbides react with water. Some - easily and without heating (for example, calcium carbide), and some (for example, silicon carbide) - when water vapor is heated to 1800 degrees. The reactivity depends on the nature of the bond in the compound, which we will talk about later. In reaction with water, various hydrocarbons are formed. This happens because the hydrogen contained in the water combines with the carbon found in the carbide. You can understand which hydrocarbon you will get (and you can get both a saturated and an unsaturated compound) based on the valency of the carbon contained in the starting substance. For example, if we have calcium carbide whose formula is CaC2, we see that it contains a C22- ion. This means that two hydrogen ions with a + charge can be attached to it. Thus, we obtain the compound C2H2 - acetylene. In the same way, from a compound such as aluminum carbide, whose formula is Al4C3, we obtain CH4. Why not C3H12, you ask? After all, the ion has a charge of 12-. The fact is that the maximum number of hydrogen atoms is determined by the formula 2n+2, where n is the number of carbon atoms. This means that only a compound with the formula C3H8 (propane) can exist, and that ion with a charge of 12- decays into three ions with a charge of 4-, which, when combined with protons, give methane molecules.
The oxidation reactions of carbides seem interesting. They can occur both when exposed to strong mixtures of oxidizing agents, and during ordinary combustion in an oxygen atmosphere. If with oxygen everything is clear: two oxides are obtained, then with other oxidizing agents it is more interesting. It all depends on the nature of the metal that is part of the carbide, as well as on the nature of the oxidizing agent. For example, silicon carbide, whose formula is SiC, when interacting with a mixture of nitric and hydrofluoric acids, forms hexafluorosilicic acid with the release of carbon dioxide. And when carrying out the same reaction, but with nitric acid alone, we obtain silicon oxide and carbon dioxide. Oxidizing agents also include halogens and chalcogens. Any carbide interacts with them; the reaction formula depends only on its structure.
The metal carbides, the formulas of which we have examined, are far from the only representatives of this class of compounds. We will now take a closer look at each of the industrially important compounds in this class and then talk about their applications in our lives.
Nitride-carbide system
In hard alloys, nitrides of metals of groups IV and V of the periodic system of elements usually appear as isomorphic satellites of the corresponding carbides.
For example, monocarbides of titanium, zirconium, vanadium, niobium and tantalum and solid solutions of these carbides often contain up to 10% nitrides in the solid solution. Systems of group IV metal carbides and group V metal nitrides (and vice versa) have been studied only recently. All of these compounds, except tantalum nitride, have the NaCl (B1) structure, and one can expect their complete mutual solubility, especially since the difference in lattice parameters is small (the exception is the ZrN-HfN-VC system). In systems involving hexagonal tantalum nitride, limited solubility should be expected. In this case, similarly to the WC–TiC system, components with a cubic lattice dissolve in the hexagonal phase in noticeable quantities; on the contrary, very low solubility at high temperatures is observed. Agte and Moepc prepared pressed mixtures of titanium and tantalum nitrides and carbides and, after partial melting, studied the structure of the resulting alloys. Duvets and Odell X-ray studied alloys obtained by high-temperature (2200-2600°) sintering in a nitrogen atmosphere of pressed mixtures of nitrides and carbides of group IV and V metals. Sintering was carried out in high-frequency or coal tube furnaces. Systems with zirconium monocarbide (since it is unstable at 2400° in a nitrogen flow) and with hafnium nitride and carbide (due to the great difficulties of obtaining them) were excluded from the study. Systems with hexagonal tantalum mononitride have also not been studied, since only limited solubility could be expected here.
Titanium nitride is titanium carbide.
Cubic crystals formed during blast furnace smelting of iron titanium ores and first described by Weller are a solid solution of 20% TiC + 80% TiN. Extensive literature is devoted to the formation of this solid solution in blast furnaces and steel-smelting furnaces and issues related to its composition. The system under consideration is of great practical importance, since all industrial titanium hard alloys contain solid solutions of titanium mononitride and monocarbide.
Agte and Moepc determined by the Pirani method the melting temperatures of various alloys of the titanium mononitride-monocarbide system. In Fig. 83 shows the results of these determinations; on the “melting point - composition” curve there is a maximum at a component ratio of 1:1.
The microstructure of such an alloy is homogeneous; X-ray diffraction studies also showed the formation of a solid solution. A continuous series of solid solutions of titanium monocarbide and mononitride was also found during X-ray studies of samples obtained by four-hour sintering at 2425°. The lattice constants as a function of composition varied linearly in accordance with Vegard's rule (Fig. 84). The measurements of Goldschmidt, who determined the lattice parameter of the 20% TiC+80% TiN solid solution to be 4.243 A, agree well with these data; specific gravity of the solid solution 5.32 g/cm3; Mooca hardness 8-8.5.
The kinetics of the reaction of titanium carbide with nitrogen was studied by Zelikman et al. Belyakova, Komar and Mikhailov observed the formation of solid solutions of TiC and TiN during the reduction of titanium dioxide with carbon in a nitrogen atmosphere at 1900°.
Jaffee, Ogden and Mikeuse studied the combined effects of carbon and nitrogen on the mechanical properties of titanium; Craighead, Simmons and Eastwood investigated the mechanical properties of Ti-Co-CN alloys melted in a vacuum arc furnace.
Titanium nitride - zirconium carbide.
Due to the active interaction of zirconium carbide with nitrogen at high temperatures, the preparation of the necessary alloys of this system is possible only at moderate temperatures and requires a long time. The difference in the lattice periods of both isomorphic components is about 10%; This also allows us to assume the presence of complete mutual solubility.
Titanium nitride - hafnium carbide.
The system has not been explored. Based on the data on the lattice parameters, the formation of solid solutions can be expected.
Titanium nitride is vanadium carbide.
Mixtures with 70-90% vanadium carbide were calcined for 2 hours at 2325°; other compositions, at 2250°. At 2100° the reaction did not proceed completely. As the results of measurements of lattice periods show (see Fig. 84), there is complete mutual solubility here.
Titanium nitride - niobium carbide.
At 2250° the process of formation of solid solutions is not completed. Briquettes sintered from mixtures of both components at 2550° (4 hours) showed complete homogeneity. The components of this system are completely mutually soluble (see Fig. 84).
Titanium nitride is tantalum carbide.
The system has not been explored. Due to the large difference in lattice sizes, significant mutual solubility cannot be expected here.
Zirconium nitride is titanium carbide.
The briquetted mixture of components did not reach equilibrium after calcination at 2425°. Blurred lines in the X-ray diffraction patterns were also shown by samples annealed at 2600° for 2 hours. or at 2650° for 1 hour. Nevertheless, the available data allow us to recognize that the components of the system form an unlimited range of solid solutions. The lattice constants as a function of composition (see Fig. 84) show a slight deviation of plus from the Begard straight line.
Zirconium nitride - hafnium carbide
(tantalum). Hafnium nitride - carbides of metals of groups IV and V. These systems have not been studied. Based on the insignificant difference in the lattice parameters of the components, it can be assumed that there is complete mutual solubility between them (with the exception of the hafnium nitride - vanadium carbide system).
Zirconium nitride - zirconium carbide
. As already indicated, this system is very difficult to study. In a nitrogen flow at temperatures below the carbide decomposition temperature, complete solubility should be expected.
Zirconium nitride is vanadium carbide.
Calcination of briquettes for 3 hours. at 2450° did not lead to interaction of the components. Their insolubility is obviously explained, as in the zirconium carbide-vanadium carbide system, by the large difference in the lattice periods.
Zirconium nitride is niobium carbide.
Contrary to the Hume-Rothery rule (the difference in lattice periods is less than 2.5%), mixtures calcined for 2 hours. at 2450°, do not form solid solutions. Obviously, this is explained by the slow diffusion of zirconium nitride. If mixtures of metal powders with carbon are used as starting materials, significant solubility can be expected.
Vanadium nitride is titanium carbide.
After 2 o'clock
After calcination at 2125°, the pressed mixtures showed complete dissolution. The values of the grating periods fit well on the Vegard straight line (Fig. 85). Vanadium nitride is zirconium carbide.
The system has not been explored. However, the components differ too greatly in their lattice constants to assume their solubility.
Vanadium nitride - hafnium carbide
(tantalum). This system has not been studied. We can assume complete mutual solubility of the components, taking into account the insignificant difference in the values of their lattice periods.
Vanadium nitride is vanadium carbide.
Pressed mixtures calcined at 2200° (2 hours) form homogeneous solid solutions. The values of the periods of their lattices lie exactly on the Vegard straight line (see Fig. 85).
Vanadium nitride is niobium carbide.
In pressed mixtures annealed at 2250°, the reaction is not completed. Also after sintering for 2 hours. at 2375° the samples produce blurred x-ray lines. Nevertheless, it can be recognized that there is complete mutual solubility due to the rectilinear nature of the “lattice constant - composition” relationship (om. Fig. 85).
Niobium nitride is titanium carbide.
The interaction of both components at 2425° is incomplete. Sharp X-ray lines are produced only by samples) calcined at 2550°. The values of the grating periods lie close to the Vegard straight line, deviating slightly to plus (Fig. 86). There is complete mutual solubility.
Niobium nitride is zirconium carbide.
Due to the instability of zirconium carbide in a nitrogen atmosphere at high temperatures, studying this system presents significant difficulties. Complete mutual solubility is possible.
Niobium nitride - hafnium carbide
(tantalum). The systems have not been explored. Due to the slight difference in the periods of the components, their complete mutual solubility can be expected.
Niobium nitride is vanadium carbide.
X-ray diffraction patterns of samples calcined for 2 hours. at 2250°, they give blurry lines. However, good agreement between the lattice parameters of various compositions and those expected by Vegard’s rule (see Fig. 86) indicates the presence of a continuous series of solid solutions in this system.
Niobium nitride is niobium carbide.
The complete mutual solubility of these components is confirmed by x-ray examination of samples calcined for 2 hours. at 2125°. The lattice constant-composition relationship is close to linear (see Fig. 86).
Tantalum nitride - carbides of metals of groups IV and V.
With the exception of the tantalum nitride - tantalum carbide system, the rest have been almost unexplored. Due to the hexagonal structure of tantalum carbide, a strong limitation or even absence of solubility can be assumed here. By analogy with the TiC-WC system, it can be assumed that cubic carbides dissolve significant amounts of hexagonal tantalum nitride, which vary significantly depending on temperature; Some solubility is also possible on the tantalum nitride side.
Data for determining the melting point of mixtures of tantalum nitride and carbide of various compositions are shown in Fig. 87. The 50:50 alloy has a two-phase structure. X-ray examination also confirms the absence of a completely homogeneous solid solution.
Recently, Campbell et al. deposits consisting of tantalum nitride and carbide were obtained by growing from the gas phase (TaCl5, nitrogen and hydrocarbons).
Uranium nitride is uranium carbide.
According to Rundle et al. this system produces a continuous series of solid solutions.
To summarize, it can be noted that in cubic systems nitride-nitride and nitride-carbide, complete mutual solubility is observed if the difference in the sizes of metal atoms does not exceed 15% (with the exception of the zirconium nitride-niobium carbide system). Solubility in nitride-carbide systems is presented schematically in Table. 65.
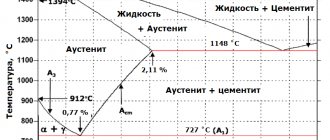
What types of carbides are there?
It turns out that carbide, the formula of which is, say, CaC2, differs significantly in structure from SiC. And the difference is primarily in the nature of the connection between atoms. In the first case, we are dealing with salt-like carbide. This class of compounds is named so because it actually behaves like a salt, that is, it is capable of dissociating into ions. This ionic bond is very weak, which makes it easy to carry out the hydrolysis reaction and many other transformations involving interactions between ions.
Another, probably more industrially important type of carbides are covalent carbides: such as, for example, SiC or WC. They are characterized by high density and strength. They are also refractory and inert to diluted chemicals.
There are also metal-like carbides. They can rather be considered as alloys of metals with carbon. Among these we can highlight, for example, cementite (iron carbide, the formula of which varies, but on average it is something like this: Fe3C) or cast iron. They have a chemical activity intermediate in degree between ionic and covalent carbides.
Each of these subtypes of the class of chemical compounds we are discussing has its own practical application. We will talk about how and where each of them is used in the next section.
Interaction of refractory metal carbides with carbon
Carbides have high melting points and hardness, increased corrosion resistance and thermal conductivity, low evaporation rates and vapor pressure at high temperatures, as well as a number of other valuable physical and technical characteristics.
Structurally, carbides are the most characteristic representatives of interstitial phases. A decrease in the melting point in the series of refractory compounds carbide—>boride—>nitride—>oxide qualitatively indicates that the bond strength in carbides is maximum. In thermodynamic terms, metal carbides of groups IV and V of the periodic table are the most stable among refractory compounds.
The development of work on the use of carbides in contact with carbon-graphite materials as protective coatings, as well as the expansion of research into the production of composite carbide-carbon materials, has aroused interest in studying the processes occurring on the carbide-carbon contact surface.
The carbon-graphite material-carbide pair is used in the designs of many high-temperature units, during the operation of which it is also necessary to take into account possible interaction at the contact boundary.
In table 35 presents the main high-temperature properties of metal carbides of groups IV-VI of the periodic system.
The refractoriness of carbides increases with increasing metal number and each group, but decreases when moving from group IV to VI.
Interacting with carbon, group V monocarbides form more refractory eutectics than group IV carbides.
Most carbides are phases of variable composition with wide regions of homogeneity. Group IV metal carbides can deviate most significantly from the stoichiometric composition. Another difference between the metals of this group is that they form only monocarbides. Metals of groups V and VI, along with monocarbides, form lower carbides of the Me2C type and have noticeably narrowed areas of homogeneity. Monocarbides of group V metals tend to form structures with a significant number of defects and achieving a stoichiometric composition is practically impossible for them. The deviation from the stoichiometric composition should facilitate diffusion due to the appearance of a significant number of vacancies in the carbon sublattice of the carbide. Indeed, the diffusion coefficient of carbon in niobium carbide of a non-stoichiometric composition is three times higher than in niobium carbide of a composition close to the stoichiometric one, but for zirconium carbide an inverse relationship is observed - with a deviation from the stoichiometric composition, the self-diffusion coefficient of carbon in zirconium carbide decreases. True, this is largely due to the fact that real group IV carbides with a carbon deficiency are usually contaminated with oxygen, the presence of which complicates the diffusion of carbon.
There is an opinion that in the case of monocarbide phases extremely saturated with carbon, the self-diffusion coefficient of carbon in the carbide of a Group IV metal is less than in the carbide of a Group V metal of the corresponding period. This is due to the fact that group IV metal carbides have less “gap” between the metal atoms both along the edge of the cube and diagonally. In addition, these carbides, corresponding to the MeC1.0 composition, have an ordered structure with a minimum number of defects, in contrast to the defective structures of Group V monocarbides. However, there is currently no clear confirmation of this assumption, but it can be said quite definitely that the coefficient of self-diffusion of carbon in carbide phases at the melting temperatures of the eutectic of MeC-C alloys increases in each group with an increase in the period by approximately an order of magnitude.
The low values of carbon diffusion coefficients in transition metal carbides allow us to recommend them as barrier layers that delay the carbidization of metals or other refractory compounds working in contact with graphite or other carbon materials.
During contact interaction between carbide and carbon, the mass transfer of carbon largely depends on the structure of the carbide material, i.e., porosity and grain size. In the presence of a significant number of communicating pores, the contribution of surface processes to the diffusion flow can be very significant. Grain boundaries have a similar effect.
When considering the processes occurring on the carbide-carbon contact surface, it is necessary to take into account that the diffusion of carbon in the carbide phase occurs much more intensely than the self-diffusion of carbon in the compared graphite. At a temperature of about 2300°K, the diffusion coefficient of carbon in carbide is several orders of magnitude higher than the self-diffusion coefficient of carbon in graphite. It follows from this that the rate of flow of carbon atoms from the bulk of graphite to the contact boundary is significantly less than the speed of their movement in the bulk of carbide. Such a difference during long-term high-temperature interaction leads to loosening of graphite in the contact zone with carbide.
During contact interaction between carbide and carbon, in addition to the melting point of the eutectic and the rate of diffusion processes, it is necessary to take into account the difference in the coefficients of thermal expansion of the materials, the strength of the adhesive bond, the influence of the environment in which the interaction occurs, and other factors.
This chapter also discusses the processes of carbide formation that occur during the interaction of molten refractory transition metals and their vapors with graphite.
The discussion of these processes, at first glance, seems somewhat outside the range of issues under consideration. However, the fact that at the melt-graphite contact boundary, as a rule, a carbide layer is formed very quickly and further interaction largely depends on the processes occurring between graphite and carbide in the solid state, makes such a discussion justified.
Knowledge of the mechanism and kinetics of interaction between graphite and carbide in the presence of a significant amount of liquid metal or its vapor in contact with graphite is necessary in practice when obtaining protective carbide coatings on graphite from melts or the vapor-gas phase, when developing technological methods for soldering graphite parts, and selecting carbon-graphite materials for melting refractory metals.
Practical application of carbides
As we have already discussed, covalent carbides have the widest range of practical applications. These include abrasive and cutting materials, and composite materials used in various fields (for example, as one of the materials included in body armor), and auto parts, and electronic devices, and heating elements, and nuclear energy. And this is not a complete list of applications of these super-hard carbides.
Salt-forming carbides have the narrowest application. Their reaction with water is used as a laboratory method for producing hydrocarbons. We have already discussed how this happens above.
Along with covalent carbides, metal-like carbides are widely used in industry. As we have already said, the metal-like type of compounds we are discussing are steels, cast irons and other metal compounds interspersed with carbon. As a rule, the metal found in such substances belongs to the class of d-metals. That is why it tends to form not covalent bonds, but, as it were, to be embedded in the structure of the metal.
In our opinion, the above compounds have more than enough practical applications. Now let's take a look at the process of obtaining them.
Preparation of carbides
The first two types of carbides that we considered, namely covalent and salt-like, are most often obtained in one simple way: the reaction of the element’s oxide and coke at high temperature. In this case, part of the coke, consisting of carbon, combines with an atom of the element in the oxide and forms a carbide. The other part “takes” oxygen and forms carbon monoxide. This method is very energy-intensive, as it requires maintaining a high temperature (about 1600-2500 degrees) in the reaction zone.
To obtain some types of compounds, alternative reactions are used. For example, the decomposition of a compound that ultimately produces carbide. The reaction formula depends on the specific compound, so we will not discuss it.
Before concluding our article, we will discuss several interesting carbides and talk about them in more detail.
Carbides
Hydrogen compounds of carbon
Chemical properties of carbon.
Under normal conditions, carbon (especially diamond) is very inert and reacts only with very energetic oxidizing agents. When heated, the chemical activity of carbon increases. In their amorphous form, coal and coke burn easily in air, producing carbon dioxide CO2. With a lack of oxygen, carbon is oxidized only to CO. Diamond can burn only in pure oxygen at 700 - 800ºС. This ability of carbon to oxidize when heated is used in the reduction of many metals from their oxides.
C + 2F2 = CF4; C + O2 = CO2; 2C + O2 = 2CO;
Carbon does not combine directly with other halogens, and the corresponding compounds are obtained indirectly. Carbon tetrachloride is prepared by passing chlorine through carbon disulfide at 60ºC in the presence of a FeS catalyst: CS2 + 2Cl2 = CCl4 + 2S.
At high temperatures, carbon reacts with sulfur, nitrogen and silicon:
C + 2S = CS2; 2C + N2 = C2N2 or (CN)2; C + Si = SiC (2000ºС).
Carbon reacts with metals at high temperatures, forming carbides. Carbides can also be obtained by reacting coal with metal oxides:
3C + CaO = CaC2 + CO.
Carbon also interacts as a reducing agent with oxides of other metals when heated: C + 2PbO = 2Pb + CO2.
Carbon dissolves only in oxidizing acids when heated:
C + 2H2SO4 (conc.) = CO2↑+ 2SO2↑+ 2H2O;
C + 4HNO3 (conc.) = CO2↑ + 4NO2↑ + 2H2O.
Hydrocarbons are quite stable, since the overlap of small valence orbitals is large, the difference in the electronegativities of carbon and hydrogen is small, and therefore strong covalent C–H bonds are formed.
Direct synthesis of methane (the simplest hydrocarbon) can only be carried out in the presence of a catalyst, for example fine nickel. The diversity of hydrocarbon compounds is explained by the ability of carbon to form endless linear and branched chains (−С−С−, >С=С< and −C≡C−) and close them into cycles with single or multiple bonds between carbon atoms both in homoatomic and in heteroatomic compounds (aromatic compounds with delocalized π bonds).
Methane itself does not react with water, acids and alkalis, and interacts with oxygen only when ignited. Unsaturated hydrocarbons are more reactive than hydrocarbons of the methane series. Their impurities can cause spontaneous combustion of swamp gas (methane) under natural conditions.
With less electronegative elements, carbon forms compounds called carbides. They can be divided into 3 groups.
Ionic-covalent carbides (salt-like) - these include methanides and acetylenides. Metanides
can be considered as methane derivatives containing the C−4 ion, for example beryllium carbide Be2C or aluminum carbide Al4C3. These are refractory crystalline substances that react with dilute acids to release methane:
Al4C3 + 12HCl = 4AlCl3 + 3CH4↑.
Acetylenides
— acetylene derivatives of the composition M+12C2, M+2C2 and M+32(C2)3, contain the C2−2 ion (where M are s- and d-metals of groups I and II of the periodic system or Al+3). Acetylenides detonate even in dry form and are decomposed by water and dilute acids:
CaC2 + 2H2O = Ca(OH)2 + C2H2.
Salt-like carbides are obtained by reacting metal oxides with graphite at high temperatures:
CaO + 3C = CaC2 + CO↑; 2 Al2O3 +9C = Al4C3 + 6CO.
Acetylenides can be obtained by the exchange interaction of acetylene with a salt of the corresponding metal.
Metal-like carbides are carbides of d-metals of groups IV – VIII of non-stoichiometric composition, which varies over a wide range; exhibit metallic properties: metallic luster, high hardness, high melting points. Carbides of titanium, vanadium, niobium, molybdenum and tungsten are also characterized by high corrosion resistance.
Metal-like carbides are produced by direct interaction of metals or their oxides with carbon in electric furnaces at high temperatures:
3Fe + C = Fe3C; V2O5 + 7C = 2VC + 5CO.
Carbides of composition M3C (where the metal has a small radius) are thermally and chemically less stable, for example, they decompose with dilute acids, releasing a mixture of hydrocarbons with fairly long chains.
Covalent carbides with an atomic crystal lattice - B4C and SiC - are products of partial replacement of carbon atoms in the diamond structure with boron or silicon atoms. Boron carbide is very hard, scratches diamond, and chemically quite inert. Carborundum is close to diamond in hardness, but is more fragile, chemically resistant and is oxidized by oxygen only at temperatures above 1000ºC. When alloyed with alkali in the presence of oxygen, carborundum is destroyed to form carbonate and silicate. SiC dissolves only in a mixture of concentrated hydrofluoric and nitric acids, in aqua regia.
Such carbides are produced in electric furnaces at very high temperatures from mixtures of SiO2 or B2O3 with coke, graphite or soot, respectively.
Application. Metal carbides give steels and cast irons hardness and wear resistance. Tungsten and tantalum carbides are used to make cutting tools and produce superhard alloys. Carborundum is used as an abrasive material, as a component of refractory materials, and as resistance rods in electric heating devices.
Hydrogen compounds of carbon
Chemical properties of carbon.
Under normal conditions, carbon (especially diamond) is very inert and reacts only with very energetic oxidizing agents. When heated, the chemical activity of carbon increases. In their amorphous form, coal and coke burn easily in air, producing carbon dioxide CO2. With a lack of oxygen, carbon is oxidized only to CO. Diamond can burn only in pure oxygen at 700 - 800ºС. This ability of carbon to oxidize when heated is used in the reduction of many metals from their oxides.
C + 2F2 = CF4; C + O2 = CO2; 2C + O2 = 2CO;
Carbon does not combine directly with other halogens, and the corresponding compounds are obtained indirectly. Carbon tetrachloride is prepared by passing chlorine through carbon disulfide at 60ºC in the presence of a FeS catalyst: CS2 + 2Cl2 = CCl4 + 2S.
At high temperatures, carbon reacts with sulfur, nitrogen and silicon:
C + 2S = CS2; 2C + N2 = C2N2 or (CN)2; C + Si = SiC (2000ºС).
Carbon reacts with metals at high temperatures, forming carbides. Carbides can also be obtained by reacting coal with metal oxides:
3C + CaO = CaC2 + CO.
Carbon also interacts as a reducing agent with oxides of other metals when heated: C + 2PbO = 2Pb + CO2.
Carbon dissolves only in oxidizing acids when heated:
C + 2H2SO4 (conc.) = CO2↑+ 2SO2↑+ 2H2O;
C + 4HNO3 (conc.) = CO2↑ + 4NO2↑ + 2H2O.
Hydrocarbons are quite stable, since the overlap of small valence orbitals is large, the difference in the electronegativities of carbon and hydrogen is small, and therefore strong covalent C–H bonds are formed.
Direct synthesis of methane (the simplest hydrocarbon) can only be carried out in the presence of a catalyst, for example fine nickel. The diversity of hydrocarbon compounds is explained by the ability of carbon to form endless linear and branched chains (−С−С−, >С=С< and −C≡C−) and close them into cycles with single or multiple bonds between carbon atoms both in homoatomic and in heteroatomic compounds (aromatic compounds with delocalized π bonds).
Methane itself does not react with water, acids and alkalis, and interacts with oxygen only when ignited. Unsaturated hydrocarbons are more reactive than hydrocarbons of the methane series. Their impurities can cause spontaneous combustion of swamp gas (methane) under natural conditions.
With less electronegative elements, carbon forms compounds called carbides. They can be divided into 3 groups.
Ionic-covalent carbides (salt-like) - these include methanides and acetylenides. Metanides
can be considered as methane derivatives containing the C−4 ion, for example beryllium carbide Be2C or aluminum carbide Al4C3. These are refractory crystalline substances that react with dilute acids to release methane:
Al4C3 + 12HCl = 4AlCl3 + 3CH4↑.
Acetylenides
— acetylene derivatives of the composition M+12C2, M+2C2 and M+32(C2)3, contain the C2−2 ion (where M are s- and d-metals of groups I and II of the periodic system or Al+3). Acetylenides detonate even in dry form and are decomposed by water and dilute acids:
CaC2 + 2H2O = Ca(OH)2 + C2H2.
Salt-like carbides are obtained by reacting metal oxides with graphite at high temperatures:
CaO + 3C = CaC2 + CO↑; 2 Al2O3 +9C = Al4C3 + 6CO.
Acetylenides can be obtained by the exchange interaction of acetylene with a salt of the corresponding metal.
Metal-like carbides are carbides of d-metals of groups IV – VIII of non-stoichiometric composition, which varies over a wide range; exhibit metallic properties: metallic luster, high hardness, high melting points. Carbides of titanium, vanadium, niobium, molybdenum and tungsten are also characterized by high corrosion resistance.
Metal-like carbides are produced by direct interaction of metals or their oxides with carbon in electric furnaces at high temperatures:
3Fe + C = Fe3C; V2O5 + 7C = 2VC + 5CO.
Carbides of composition M3C (where the metal has a small radius) are thermally and chemically less stable, for example, they decompose with dilute acids, releasing a mixture of hydrocarbons with fairly long chains.
Covalent carbides with an atomic crystal lattice - B4C and SiC - are products of partial replacement of carbon atoms in the diamond structure with boron or silicon atoms. Boron carbide is very hard, scratches diamond, and chemically quite inert. Carborundum is close to diamond in hardness, but is more fragile, chemically resistant and is oxidized by oxygen only at temperatures above 1000ºC. When alloyed with alkali in the presence of oxygen, carborundum is destroyed to form carbonate and silicate. SiC dissolves only in a mixture of concentrated hydrofluoric and nitric acids, in aqua regia.
Such carbides are produced in electric furnaces at very high temperatures from mixtures of SiO2 or B2O3 with coke, graphite or soot, respectively.
Application. Metal carbides give steels and cast irons hardness and wear resistance. Tungsten and tantalum carbides are used to make cutting tools and produce superhard alloys. Carborundum is used as an abrasive material, as a component of refractory materials, and as resistance rods in electric heating devices.
Interesting connections
Sodium carbide. The formula of this compound is C2Na2. It can be thought of as an acetylenide (that is, the product of the replacement of hydrogen atoms in acetylene with sodium atoms) rather than a carbide. The chemical formula does not fully reflect these subtleties, so they must be looked for in the structure. This is a very active substance and upon any contact with water it very actively interacts with it to form acetylene and alkali.
Magnesium carbide. Formula: MgC2. Interesting ways to obtain this fairly active compound. One of them involves sintering magnesium fluoride with calcium carbide at high temperature. As a result, two products are obtained: calcium fluoride and the carbide we need. The formula for this reaction is quite simple, and you can read it in specialized literature if you wish.
If you are not sure of the usefulness of the material presented in the article, then the next section is for you.
What is carbide. Properties of carbide. Application of carbide
Childhood fun. This is how many people remember carbide, especially former boys, and now, of course, adult men.
They took stones from construction markets. They didn’t buy it, but instead dragged it from dump stations and from the backs of trucks.
The prey was placed in bottles, filled with water, closed, and shaken. All that was left was to throw the container and admire the explosion.
We also admired the whitish bubbles that carbide gave when falling into puddles. However, to whom they owe such fun, the tomboys of yesteryear often did not know.
What is carbide ? Let's try to answer a question that seemed unimportant in childhood.
What is carbide
Carbite is not a specific substance, but a group of compounds of elements with carbon. The latter must be more electronegative than its “neighbor”.
This is a mandatory condition that excludes carbon halides and oxides from the range of carbides.
Electronegativity refers to the ability of an atom to shift electrons of other substances towards itself.
The electronegativity of carbon is 2.6. This is the Pauling scale data. It is built taking into account that ionicity in a covalent bond makes this bond stronger.
It turns out that the electronegativity of the second elements in carbides should be less than 2.6.
Most suitable elements are metals. But about 15% of carbides do not contain them.
Externally, carbides are crystalline, usually colorless, transparent substances. They have a diamond shine.
The compounds owe them to carbon, which is the basis of not only carbides, but also diamonds.
In fact, the heroes of the article are diamonds in which some of the atoms are replaced by other elements.
There are also colored duets with carbon, for example, iron carbide . This is the familiar cement. The color of the connection is gray.
It turns out that the properties of carbides may vary. We will consider discrepancies in the chapter “Types”. For now, let's study the general characteristics of the class of compounds.
Carbide properties
The general properties of carbides include hardness. It may be more or less, but always above average.
Some representatives of the group have an indicator close to corundum and diamond. These are the hardest minerals on earth.
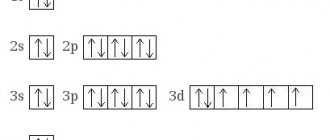
Transition metal carbides were especially distinguished. These are elements of side subgroups of the periodic table. All transition metals have electrons in d and f orbitals.
Summarizes carbides and high melting point. As a rule, it is higher than that of the metal included in the connection.
If it is transitional, softening can begin only at 3000 degrees Celsius.
It is interesting that the melting point rises along with the number of the group to which the “neighbor” of carbon belongs.
The most refractory are carbides with elements from groups 5-7 of the periodic table.
Where carbide can be understood by the structure of the compounds. Their grilles are often defective.
This means that there are deviations from the theoretical scheme, breaks and displacements. This is why the properties of carbides can differ 100 or even 1000 times from those calculated using formulas.
Thus, many compounds of the class are resistant to corrosion and are insoluble in most acids.
Types of carbides
There are three main types of carbides. The first is covalent compounds. Valence is a predisposition to a certain number of chemical bonds.
A covalent bond is the overlap of valence clouds of different elements. That is, they form common electron pairs. These are the ones that form the basis of covalent carbides.
Covalent carbides include carbides of only two elements: bromine and silicon. Both compounds are chemically inert. Their interatomic bonds are strong.
As a result, the carbides of the group are difficult to melt - the lattice does not want to collapse. The strength of the bond makes both connections solid.
Bromine carbide even rivals diamond. Some carbon samples scratch diamonds, meaning they are harder than diamonds.
Silicon carbide does not “win” diamond, but it has its own decent 8 points on the Mohs scale.
Only hydrofluoric acid, concentrated nitric and aqua regia dissolve covalent carbides. Oxidation of carbides of the group occurs only when heated to 1000 degrees.
The second type of carbides is ionic. It is also called salt-like. All are formed by metals of the 1st and 2nd groups of the periodic table.
The class also includes aluminum carbide . Compounds of the group are decomposed not only by acids, but also by water.
Pebbles that make puddles “boil,” for example, are calcium carbide . By the way, it is quite toxic and can corrode mucous membranes. Why it is brought to construction markets, we will understand in the next chapter.
When ionic carbides react with water, hydrogen is released. Metal hydroxide forms and precipitates in the liquid.
The reaction proceeds rapidly. A sudden release of hydrogen onto the surface of the water produces that same bubbling.
The third type of carbides is ionic-covalent-metallic, simply metal-like.
Such compounds are formed by elements of the 4th, 5th, 6th, 7th groups of the periodic table. Exceptions: - nickel, cobalt and iron carbides.
If covalent carbides have low chemical activity, and ionic carbides have high chemical activity, then the third type of compound has average activity.
The molecular structure is remarkable. Their basis is metal atoms. Carbon atoms are located in the voids between them.
Therefore, for example, tungsten carbide is called embedded. This means that carbon has penetrated into the crystal lattice of the metal.
This structure provides record strength and a high melting point. Another well-known compound of the group is titanium carbide .
Application of carbide
Titanium carbide has become the basis for tungsten-free but equally hard alloys.
In addition, the compound serves as a coating for tools, mainly industrial and construction.
This spraying minimizes the wear of parts and allows them to process even the hardest materials.
Silicon carbide is also used as an abrasive. In its natural form, which is the mineral moissanite, the compound is valued by jewelers, moreover, higher than cubic zirconia, which is similar in appearance and properties.
Calcium carbide is needed for welding work. is obtained from the compound . Carbide serves as its source, and at the same time, as fuel for oxygen welding machines.
Acetylene is a gas. One is enough to operate the devices. But there is also water. Calcium carbide reacts violently with it.
The result is not only bubbles that children like, but also an abundance of heat - another source of energy.
Boron carbide is used as a refractory. The melting point of the compound is almost 2500 degrees.
The strength of carbide allows it to be added to body armor. The material can protect not only from bullets, but also from radiation.
Therefore, one of the answers to the question of where to get boron carbide is in protective screens that block radiation.
The list of carbides and their role in society could take many pages. There are several dozen compounds and each of them has a use, and more than one. There is no single scheme for producing carbides.
We will have to limit ourselves to general phrases. However, they also contain a lot of useful information.
Preparation of carbides
Most carbides are produced rather than mined. The first synthesis was carried out at the beginning of the 19th century.
An Englishman named Davy obtained potassium carbide . In 1863, copper carbide was created.
It turned out to be unstable, unlike the third synthesized carbon-iron compound.
Looking at the prototypes, scientists could not figure out where to find carbide outside the laboratories.
Minerals in which metals are combined with carbon were discovered only at the beginning of the 20th century.
In addition to moissanite, geologists found cohenite - a mixture of cobalt carbide with nickel and iron.
Judging by the date of discovery of carbide minerals, they are not common.
Therefore, on an industrial scale, the heroes of the article are still being synthesized. The mass of carbide can be obtained, for example, from charcoal and metal oxides.
They are converted into carbides using a voltaic arc and an electric furnace.
Carbide price
to buy calcium carbide for about 40-90 rubles per kilogram. The combination of carbon and boron costs from 100 rubles per kilo.
to buy silicon carbide for about 160 rubles per 1000 grams.
But for a kilo of hafnium carbide you will have to pay about 21,000 rubles, moreover, for wholesale purchases.
That is, the cost of the material largely depends on the metal or non-metal present in it. There is even gold carbide .
By the way, it is capable of exploding simply by pouring powder. So, even at a high price, delivering raw materials to the consumer is not an easy task.
How can this be useful in life?
Well, firstly, knowledge of chemical compounds can never be superfluous. It is always better to be armed with knowledge than to be left without it. Secondly, the more you know about the existence of certain compounds, the better you understand the mechanism of their formation and the laws that allow them to exist.
Before moving on to the end, I would like to give some recommendations for studying this material.