Copper(I) oxide
| Names | |
| IUPAC Name Copper(I) Oxide | |
| Other names Copper oxide Copper oxide Copper oxide Cuprite Red copper oxide | |
| Identifiers | |
| Number of CAS |
|
| 3D model (JSmol) |
|
| CHEBY |
|
| ChemSpider |
|
| ECHA InfoCard | 100,013,883 |
| EU number |
|
| KEGG |
|
| PubChem C.I.D. |
|
| RTECS number |
|
| UNII |
|
| CompTox Dashboard (EPA) |
|
InCHI
| |
Smiles
| |
| Characteristics | |
| Chemical formula | Cu2O |
| Molar mass | 143.09 g/mol |
| Appearance | brownish-red solid |
| Density | 6.0 g/cm3 |
| Melting temperature | 1232 °C (2250 °F, 1505 K) |
| Boiling point | 1800 °C (3270 °F, 2070 K) |
| Solubility in water | Insoluble |
| Acid solubility | Soluble |
| Bandgap | 2,137 |
| Magnetic susceptibility (χ) | −20 × 10 −6 cm 3 / mol |
| Structure | |
| Crystal structure | cubic |
| Space group | Mon 3 m, #224 |
| Lattice constant | A = 4,2696 |
| Thermochemistry | |
| Standard molar entropy ( S o 298) | 93 J mol −1 K −1 |
| Std formation enthalpy (Δ F H ⦵ 298 ) | -170 kJ mol -1 |
| Dangers | |
| MSDS | SIRI.org |
| GHS Pictograms | |
| GHS signal word | Danger |
| GHS Hazard Statements | H302, H318, H332, H400, H410 |
| GHS Precautions | P273, P305 + 351 + 338 [1] |
| NFPA 704 (fire diamond) | 0 2 1 |
| NIOSH (US Health Exposure Limits): | |
| PEL (Permissible) | TWA 1 mg/m3 (as Cu) [2] |
| REL (recommended) | TWA 1 mg/m3 (as Cu) [2] |
| IDLH (Imminent Hazard) | TWA 100 mg/m3 (as Cu) [2] |
| Related compounds | |
| Other anions | Copper(I) sulfide Copper(II) sulfide Copper(I) selenide |
| Other cations | Copper(II) oxide Silver(I) oxide Nickel(II) oxide Zinc oxide |
| Unless otherwise stated, data is for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Y check (what is ?)YN | |
| Links to infoboxes | |
Copper(I) oxide
or
copper oxide
is an inorganic compound with the formula Cu 2 O. It is one of the main oxides of copper, the other is either copper(II) oxide or copper oxide (CuO). This red solid is found in some antifouling paints. Depending on the particle size, the compound may appear yellow or red. [3] Copper(I) oxide occurs as the reddish mineral cuprite.
Preparation[edit]
Copper(I) oxide can be produced in several ways. [4] Simply put, it results from the oxidation of copper metal:
4 Cu + O 2 → 2 Cu 2 O
Additives such as water and acids affect the rate of this process, as well as further oxidation to copper(II) oxides. It is also produced on an industrial scale by reducing copper(II) solutions with sulfur dioxide. Aqueous solutions of cuprous chloride react with a base to form the same material. In all cases, color is very sensitive to the details of the procedure.
Pourbaix diagram for copper in uncomplexed media (except for OH - anions - is not taken into account). Ion concentration 0.001 mol/kg water. Temperature 25°C.
The formation of copper(I) oxide is the basis of the Fehling and Benedict tests for reducing sugars. These sugars reduce the alkaline copper(II) salt solution, giving a bright red precipitate of Cu2O.
It forms on silver-plated copper parts exposed to moisture when the silver layer is porous or damaged. This type of corrosion is known as red plague.
There is little evidence for the existence of copper(I) hydroxide CuOH, which is expected to undergo dehydration quickly. A similar situation applies to gold(I) and silver(I) hydroxides.
Receipt
Copper(I) oxide can be prepared:
- heating copper metal in the absence of oxygen
4Cu + O2 →>200∘C 2Cu2O
- heating copper metal in a stream of nitric oxide (I) or nitric oxide (II)
2Cu + N2O →500−600∘C Cu2O + N2 4Cu + 2NO →500−600∘C 2Cu2O + N2
- heating copper metal with copper(II) oxide
Cu + CuO →1000−1200∘C Cu2O
- thermal decomposition of copper (II) oxide
4CuO →1026−1100∘C 2Cu2O + O2
- heating copper(I) sulfide in a stream of oxygen
2Cu2S + 3O2 →1200−1300∘C 2Cu2O + 2SO2
In laboratory conditions, copper (I) oxide can be obtained by reducing copper (II) hydroxide (for example, with hydrazine):
4Cu(OH)2 + N2H4 ⋅ H2O →100∘C 2Cu2O ↓ + N2↑ + 7H2O
Also, copper(I) oxide is formed in ion exchange reactions of copper(I) salts with alkalis, for example:
- in the reaction of copper (I) iodide with a hot concentrated solution of potassium hydroxide
2CuI + 2KOH ⟶ Cu2O↓ + 2KI + H2O
- in the reaction of hydrogen dichlorocuprate (I) with a dilute solution of sodium hydroxide
2H[CuCl2] + 4NaOH ⟶ Cu2O↓ + 4NaCl + 3H2O
In the last two reactions, a compound with a composition corresponding to the formula CuOH (copper (I) hydroxide) is not formed. The formation of copper (I) oxide occurs through an intermediate hydrate form of variable composition Cu2O ⋅ xH2O.
- Oxidation of aldehydes with copper (II) hydroxide. If an aldehyde solution is added to the blue precipitate of copper (II) hydroxide and the mixture is heated, then a yellow precipitate of copper (I) hydroxide appears first:
R−CHO + 2Cu(OH)2 →t R−COOH + 2CuOH↓ + H2O upon further heating, the yellow precipitate of copper (I) hydroxide turns into red copper (I) oxide: 2CuOH →tCu2O + H2O
Properties [edit]
A solid is diamagnetic. From the point of view of their coordination spheres, the copper centers are bicoordinated, and the oxides are tetrahedral. Thus, the structure is in some sense reminiscent of the basic SiO 2 polymorphs, and both structures have interpenetrating lattices.
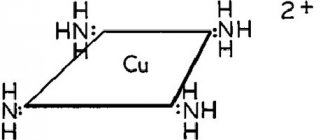
Copper(I) oxide dissolves in a concentrated ammonia solution to form a colorless complex +, which easily oxidizes in air to a blue color 2+. . It dissolves in hydrochloric acid to form solutions of CuCl.-2. Dilute sulfuric acid and nitric acid produce copper(II) sulfate, and copper(II) nitrate, respectively. [5]
Cu 2 O decomposes to copper (II) oxide in humid air.
Ammonia solution of copper oxide formula
Copper oxide ( I) Cu2 O -
reddish-brown crystals with a cubic crystal lattice, in which linear-tetrahedral coordination of atoms is realized, density 6.1 g/cm 3, melting point 1242°C.
It does not dissolve in water and does not react with it. It has weakly expressed amphoteric properties with a predominance of basic ones.
Interacts with alkali solutions to form hydroxo complexes:
In aqueous solutions of ammonia it forms diammine copper (I) hydroxide:
Reacts with hydrochloric acid to form hydrogen dichlorocuprate (I):
With hydrogen bromide and hydrogen iodide it forms copper (I) salts:
In dilute sulfuric acid it disproportions, forming copper (II) sulfate and metallic copper:
Reduced by hydrogen, carbon monoxide and active metals to metallic copper:
When heated, it is oxidized by atmospheric oxygen:
Copper(I) oxide is produced by electrolysis of a sodium chloride solution using copper electrodes. Hydrogen is released at the cathode, and copper dissolves at the anode to form Cu + ions, and upon interaction with OH groups, Cu2O is formed.
Copper (I) oxide is formed when copper (II) oxide is heated to 1100°C:
or when reducing copper sulfate with glucose or hydrazine in an alkaline medium
:
Copper hydroxide ( I)
CuOH
as an individual compound has not been isolated. When copper(I) salts interact with alkalis in solution, hydrated oxide Cu2O · nH2O is formed, and only Cu2O is released from the solution. When Cu2O is dissolved in alkali solutions, M[Cu(OH)2] is formed.
Source
Semiconductor properties[edit]
In the history of semiconductor physics, Cu2O is one of the most studied materials, and many experimental applications of semiconductors were first demonstrated in this material:
- Semiconductor
- Semiconductor diodes [6]
- Phonoritons ("coherent superposition of exciton, photon and phonon") [7][8]
The lowest excitons in Cu 2 O are extremely long-lived; the shape of the absorption lines has been demonstrated with a neV linewidth that is the narrowest bulk exciton resonance ever observed. [9] The corresponding quadrupole polaritons have a low group velocity, approaching the speed of sound. Thus, in this environment, light travels almost as slowly as sound, resulting in a high density of polaritons. Another unusual feature of ground state excitons is that all primary scattering mechanisms are known quantitatively. [10] Cu 2 O was the first substance in which a completely parameter-free model of absorption linewidth broadening with temperature could be established, allowing the corresponding absorption coefficient to be derived. Using Cu 2 O, it can be shown that the Kramers–Kronig relations do not apply to polaritons. [eleven]
Ammonia solution of copper oxide formula
Copper oxide ( II)
CuO
- black crystals, crystallizes in a monoclinic system, density 6.51 g/cm 3, melting point 1447 ° C (under oxygen pressure). When heated to 1100°C, it decomposes to form copper (I) oxide:
It does not dissolve in water and does not react with it. It has weakly expressed amphoteric properties with a predominance of basic ones.
In aqueous solutions of ammonia it forms tetraammine copper (II) hydroxide:
Reacts easily with dilute acids to form salt and water:
When fused with alkalis it forms cuprates:
Reduced by hydrogen, carbon monoxide and active metals to metallic copper:
It is obtained by calcining copper (II) hydroxide at 200°C:
or during the oxidation of copper metal in air at 400–500°C:
Copper (II) hydroxide Cu(OH)2
– a blue substance, exists in amorphous and crystalline forms, the crystal lattice is rhombic, density 3.37 g/cm 3, when heated above 70°C, it decomposes into copper (II) oxide and water:
Poorly soluble in water. It has weakly expressed amphoteric properties with a predominance of basic ones.
Reacts easily with acids to form salts:
In aqueous solutions of alkalis it forms unstable bright blue hydroxo complexes:
In ammonia solution there are stable ammonia compounds of dark blue color:
Displaying basic properties, it interacts with carbon dioxide to form the main copper (II) carbonate – malachite:
Obtained by the exchange interaction of copper (II) salts and alkali
:
crystalline copper (II) hydroxide is formed by introducing sodium or potassium hydroxide into an ammonia solution of copper (II) sulfate:
Source
Links[edit]
- https://www.nwmissouri.edu/naturalsciences/sds/c/Copper%20I%20oxide.pdf
- ^ abc NIOSH Pocket Guide to Chemical Hazards. "#0150" . National Institute of Occupational Safety and Health (NIOSH).
- N.N. Greenwood, A. Earnshaw, Chemistry of the Elements
, 2nd ed., Butterworth-Heinemann, Oxford, UK, 1997. - H. Wayne Richardson "Copper Compounds in Ullman's Encyclopedia of Industrial Chemistry 2002, Wiley-VCH, Weinheim. Doi: 10.1002 / 14356007.a07_567″
- D. Nicholls, First Line Complexes and Transitions
, Macmillan Press, London, 1973. - L.O. Grondal, Unidirectional current device, patent, 1927
- Hanke, L.; Fröhlich, D.; Ivanov, AL; Littlewood, PB; Stolz, H. (1999-11-22). "LA Phonoritons in Cu 2 O". Physics Review Letters
.
83
(21):4365–4368. DOI: 10.1103/PhysRevLett.83.4365. - L. Brillouin: Wave Propagation and Group Velocity
, Academic Press, New York, 1960 ISBN 9781483276014. - J. Brandt, D. Fröhlich, K. Sandfort, M. Bayer, H. Stolz and N. Naka, Ultranarrow absorption spectroscopy and two-phonon excitation of Cu 2 O paraexcitons in a strong magnetic field
, Phys. Rev. Lett. 99, 217403 (2007). DOI: 10.1103/PhysRevLett.99.217403 - J. P. Wolf and A. Mysyrowicz: Exciton Substance, Scientific American 250
(1984), No. 3, 98. - Hopfield, J. J. (1958). "Theory of the contribution of excitons to the complex dielectric constant of crystals." Physical Review
.
112
(5):1555–1567. DOI: 10.1103/PhysRev.112.1555. ISSN 0031-899X. - https://www.mindat.org/min-3098.html
- https://www.ima-mineralogy.org/Minlist.htm
Copper(I) oxide
| Copper(I) oxide | |
| Traditional names | Cuprous oxide, cuprous oxide, dicopper oxide |
| Chem. formula | Cu2O |
| Rat. formula | Cu2O |
| Appearance | Brown-red crystals |
| Molar mass | 143.09 g/mol |
| Density | 6.1 g/cm³ |
| Hardness | 3,5 — 4 |
| Enthalpy | |
| • melting | +64.22 kJ/mol |
| Solubility | |
| • in water | 2.4⋅10 −7 g/100 ml |
| Refractive index | 2,85 |
| Crystal structure | cubic |
| Reg. CAS number | 1317-39-1 |
| PubChem | 10313194 |
| Reg. EINECS number | 215-270-7 |
| SMILES | |
| RTECS | GL8050000 |
| ChEBI | 81908 |
| ChemSpider | 8488659 |
| LD50 | 470 mg/kg |
| Toxicity | average |
| GHS pictograms | |
| Data given is based on standard conditions (25 °C, 100 kPa) unless otherwise stated. | |
Copper(I) oxide
(copper hemioxide, dicopper oxide,
obsolete
copper oxide) is a chemical compound with the formula Cu2O. Compound of copper with oxygen, basic oxide. Brown-red crystalline substance. It occurs in nature as the mineral cuprite.
COPPER OXIDES
COPPER OXIDES, chemical compounds of copper with oxygen, corresponding to decomp. oxidation states of copper. Oxide $\ce{Cu(I) Cu2O}$ (hemioxide) – reddish-brown crystalline. substance with $t_{pl}$ 1242 °C. In nature, the mineral cuprite. The oxide $\ce{Cu(I)}$ is practically insoluble in water; when interacting with aqueous solutions of alkalis, it forms complex hydroxides of the composition $\ce{M^{I}[Cu^{I}(OH)2]}$ ($\ce{M}$ – alkali metal); is restored when heated to metallic. $\ce{Cu}$ (e.g. hydrogen, $\ce{C, CO}$); when heated, it is oxidized by oxygen to $\ce{CuO}$; with dilute $\ce{H2SO4}$ forms $\ce{Cu and CuSO4}$, in aqueous solutions of $\ce{NH3}$ – ammonia $\ce{[Cu(NH3)2]OH}$. $\ce{Cu(I)}$ hydroxide has not been isolated as an individual compound. $\ce{Cu(I)}$ oxide is obtained by electrolysis of a $\ce{NaCl}$ solution using copper electrodes; used as a pigment for coloring glass, ceramics, enamels, as a component of paints, in the village. x-ve - as a fungicide.
Oxide $\ce{Cu(II) CuO}$ – black crystalline. substance with $t_{pl}$ 1447 °C (under pressure $\ce{O2}$); in nature – the mineral tenorite. $\ce{Cu(II)}$ oxide is practically insoluble in water; begins to decompose at a temperature of 800 °C, at 1100 °C it decomposes completely to $\ce{Cu2O\: and\: O2}$; is restored when heated to metallic. $\ce{Cu}$ (e.g. hydrogen, $\ce{C, CO}$); dissolves in acids (used in copper hydrometallurgy); in aqueous solutions $\ce{NH3}$ forms ammonia $\ce{[Cu(NH3)4](OH)2}$, when fused with alkalis it forms $\ce{M^{I}CuO2}$ cuprates. $\ce{Cu(II)}$ oxide is prepared by reacting $\ce{CuSO4}$ sulfate with either $\ce{NaOH}$ or $\ce{KOH}$ at 80–90 °C, or with an aqueous solution of $ \ce{NH3}$ followed by decomposition at 200 °C of the resulting hydroxide $\ce{Cu(OH)2}$; used to produce oxide catalysts, as a pigment for glass, ceramics, enamels, and in electroplating - for the preparation of electrolytes.
The oxide $\ce{Cu(II)}$ corresponds to the hydroxide $\ce{Cu(OH)2}$ - greenish-blue amorphous or crystalline. a substance that is unstable and practically insoluble in water. Hydroxide $\ce{Cu(OH)2}$ exhibits amphoteric properties (the acidic character is weakly expressed): it dissolves in acids to form the corresponding salts $\ce{Cu(II)}$ and in concentrated. aqueous solutions of alkalis with the formation of $\ce{M^{I}_2[Cu(OH)4]}$ cuprates. In an aqueous solution of ammonia, $\ce{Cu(OH)2}$ forms a blue solution $\ce{[Cu(NH3)4](OH)2}$, capable of dissolving cellulose (used in the production of copper-ammonia fibers). Hydroxide $\ce{Cu(OH)2}$ is obtained by reacting salts $\ce{Cu}$ with alkalis in an aqueous solution; used to obtain $\ce{Cu(II)}$ salts, as a mordant for dyeing, as a pigment for glass, enamels, glazes, a fungicide, etc.
Oxide $\ce{Cu(III) Cu2O3}$ (sesquioxide) – dark red substance; obtained by the action of $\ce{K2S2O8}$ on $\ce{Cu(OH)2}$ in an alkaline medium; is a strong oxidizing agent, decomposes at 400 °C to $\ce{CuO}$ and $\ce{O2}$, forms cuprates(III) $\ce{M^{I}[Cu(OH)4] with alkali solutions }$.
M. o. and their derivatives are toxic.