Historical reference
Copper is one of the most important elements of antiquity. Copper, gold , silver and tin were the first metals that humanity recognized in its historical development. Because copper is easily processed, it was used by ancient cultures more than 10,000 years ago. The time of widespread use of copper began in the 5th millennium BC. to the 3rd millennium BC
However, in its pure form, copper turned out to be relatively soft for the production of weapons and tools. Therefore, ancient people, through experiments, adding pieces of lead and tin to molten copper, obtained bronze. It is a much harder material than unalloyed copper. Bronze has been used by humanity for over 5,000 years. This alloy gave its name to an entire historical era.
In alchemy, copper was associated with Venus (femininity). Of course, not least because the first mirrors used by women were made of this metal.
Physical properties
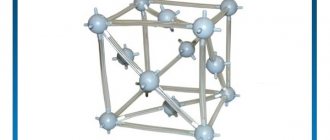
With a density of 8920 kg/m3, copper is one of the heavy metals with a melting point of 1083.4 C. It crystallizes into the face-centered cubic (FCC) system and has a Mohs hardness of 2.5 to 3. Copper is a very good conductor of electricity. Slightly worse than silver, and much better than gold. In addition, copper is a very good conductor of heat.
However, aluminum is a better electrical conductor per gram of substance than copper. But it is more voluminous, so copper per square centimeter of cable cross-section conducts electricity better than the same cross-section of aluminum wire.
Pure metallic copper has a bright red color with a pink tint. When exposed to air, copper takes on a reddish-brown hue. Due to further oxidation and corrosion, a patina develops very slowly (often over centuries) on the surface of the copper. The metallic luster is lost and the color changes from reddish-brown to bluish-green.
Chemical structure and properties
If you study the electronic formula of the copper atom, you will find that it has 4 levels. There is only one electron in the 4s valence orbital. During chemical reactions, from 1 to 3 negatively charged particles can be separated from one atom, resulting in copper compounds with an oxidation state of +3, +2, +1. Its divalent derivatives are the most stable.
In chemical reactions it acts as an inactive metal. Under normal conditions, copper does not dissolve in water. In dry air, corrosion is not observed, but when heated, the metal surface becomes covered with a black coating of divalent oxide. The chemical stability of copper is manifested under the influence of anhydrous gases, carbon, numerous organic compounds, phenolic resins and alcohols. It is characterized by complex formation reactions with the release of colored compounds. Copper has little in common with the metals of the alkaline group, associated with the formation of monovalent derivatives.
Sources
- https://vseowode.ru/prosto-o-vode/rastvorimost-v-vode-medi.html
- [https://ollimpia.ru/v-kakih-zhidkostyah-mozhno-rastvorit-med/]
- [https://morflot.su/v-chem-mozhno-rastvorit-med/]
- [https://scienceforyou.ru/teorija-dlja-podgotovki-k-egje/himicheskie-svojstva-perehodnyh-metallov]
- [https://FB.ru/article/238897/rastvorimost-medi-v-vode-i-kislotah]
Copper deposits
Copper sometimes occurs in nature in its pure form as a solid element. Mainly in basaltic lavas. It is found there in the form of a nugget (solidified melt) or in branched rock structures, the so-called dendrites, very rarely in crystalline form. The proportion of pure copper in nature is very low.
In contrast, copper ores are very common. Copper is mined from the following minerals: chalcopyrite (copper gravel - CuFeS2), chalcocite (copper gloss - Cu2S), less commonly from bornite (Cu5 FeS4), atacamite, malachite and others. The largest copper deposits in the world are located in Chile, USA, Russia, Zambia, Canada and Peru.
The main copper producing country is Chile, followed by Indonesia and the United States. The main exporting countries are united in the Commonwealth of Producing Countries - CIPEC. CIPEC includes Chile, Peru, Australia, Indonesia, Democratic Republic of Congo and Papua New Guinea.
Dissolution in ammonia
This process often occurs by passing NH3 in gaseous form over cast iron metal. The result is the dissolution of copper in ammonia, the release of Cu3N. This compound is called monovalent nitride.
Its salts are exposed to ammonia solution. Adding this reagent to copper chloride results in a precipitate of hydroxide:
Excess ammonia promotes the formation of a complex type of compound that has a dark blue color:
This process is used to determine cupric ions.
Copper production
To produce copper from copper gravel (CuFeS2), the so-called copper stone (Cu2S with various FeS contents) with a copper content of about 70% is initially obtained. To do this, the source material is heated with the addition of coke and the iron oxides it contains, slagged with siliceous fillers. The resulting iron silicate slag floats in the melt on the surface and can be easily drained. The copper stone is further processed into raw copper ( black copper ) with a copper content of about 98%.
To do this, the melt is poured into the converter and air is blown in. In the first stage (slag blowing), the iron sulfide contained in it is fried into iron oxide, and the flaked quartz binds to the slag, which can be drained. In the second stage, two thirds of the remaining Cu2S is oxidized to Cu2O. The oxide then reacts with the remaining sulfide to form crude copper. The raw copper ( cement copper ) is then refined electrolytically.
Copper migrates as ions through the electrolyte to the cathode and is deposited there. The final copper content is 99.99% with very little admixture of other substances. The less noble metals of these impurities remain dissolved in the electrolyte; the more noble metals (including silver and gold) form an “electrolyte precipitate” and are further processed separately.
Copper Applications
The modern market offers a wide range of consumer products containing copper: from dishes to computers. Copper is used to make coins, electrical wires, jewelry, cutlery, fittings, teapots, precision parts, artwork, musical instruments, piping and more.
For electrical conductive cables and lines, printed circuit boards and integrated circuits, electrical components (transformer windings, inductance chokes, magnetron anode bodies) only pure copper is used due to its very good electrical conductivity. Beryllium copper is used for overhead lines.
Copper is highly reflective in the infrared range and is therefore used as mirrors for carbon dioxide laser systems. Due to its good thermal conductivity, copper is often used as thermal radiators.
Copper is part of many alloys, such as golden yellow brass (with zinc ), bronze (with tin), and nickel-plated silver (with zinc and nickel ). Wrought alloys (brass and nickel-plated silver) are brought into the desired shape by plastic forming (hot forming: rolling, forging or cold forming: wire drawing, forging, cold rolling, deep drawing), while cast materials (gun steel, bronze ) is usually difficult or impossible to mold plastically.
Objects with a silvery-white (stainless steel-like) appearance are often actually high-copper alloys, since the color of the copper disappears completely when nickel is added. Modern coins are made from an alloy of copper, zinc, aluminum and tin. Copper compounds are used in color pigments, as toners, medicines and electroplating. Thanks to its noble appearance, copper is indispensable in the furniture industry and in the field of decoration.
Solubility in mercury
When mercury is mixed with metals of other elements, amalgams are obtained. This process can occur at room temperature because Pb is a liquid under such conditions. The solubility of copper in mercury disappears only when heated. The metal must first be crushed. When solid copper is wetted with liquid mercury, one substance penetrates or diffuses into the other. The solubility value is expressed as a percentage and is 7.4 * 10 -3. The reaction produces a strong and simple amalgam, similar to cement. If you warm it up a little, it will become soft. As a result, this mixture is used to repair porcelain products. There are also complex amalgams with optimal metal content. For example, a dental alloy contains the elements silver, tin, copper and zinc. Their percentage is 65: 27: 6: 2. A mixture with this composition is called silver. Each component of the alloy performs a specific function to achieve a high-quality seal.
Another example is an amalgam alloy, which has a high copper content. It is also called copper alloy. Amalgam contains from 10 to 30% Cu. The high copper content prevents the tin from reacting with the mercury, which prevents the formation of a very weak and corrosive alloy phase. In addition, reducing the amount of silver in the filling leads to lower costs. To prepare amalgam, it is recommended to use an inert atmosphere or a film-forming protective liquid. The metals that make up the alloy can be quickly oxidized by air. The process of heating copper amalgam in the presence of hydrogen removes the mercury, allowing the elemental copper to be separated. As you can see, mastering this topic is not difficult. Now you know how copper interacts not only with water, but also with acids and other elements.
Biological effect
Copper is a component of blue hemocyanin, which is used by many molluscs and arthropods as a blood dye to transport oxygen. Copper is also a vital trace element in higher organisms and is a component of many enzymes.
The daily requirement of copper for an adult is about 2 milligrams.
The copper depot in the human body is located in the liver. Excess copper is excreted through the digestive system along with bile. Compared to many other heavy metals, an excess of copper does not cause significant harm to the body. A person can eat 0.04 grams of copper per day without harming their health. Copper is mainly found in chocolate, liver, cereals, vegetables and nuts.
Copper deficiency is rarely diagnosed in people. It is mainly observed in chronic diarrhea, in premature babies, and during prolonged fasting. Consuming high doses of zinc, iron , or molybdenum can reduce the amount of copper in the body. In its free (not bound to protein) form, copper has pronounced antibacterial properties. Pure silver has the same qualities.
Copper sulfate ( copper sulfate ) is a strong emetic and is therefore used to treat many intoxication diseases at the acute stage.
Solubility in sulfuric acid
Under normal conditions, this reaction does not occur. The factor determining the dissolution of copper in sulfuric acid is its strong concentration. A dilute medium cannot oxidize the metal. The dissolution of copper in concentrated sulfuric acid proceeds with the release of sulfate.
The process is expressed by the following equation:
Cu + H2SO4 + H2SO4 → CuSO4 + 2H2O + SO2.
Copper and water
The average concentration of copper in seawater is about 0.2–3 ppb, although values can vary greatly. River water is typically 2-5 ppb. Kelp contains about 2-68 ppm (dry matter), while oysters contain about 63 ppm. In the dissolved state, the element is in the form of CuOH + or in the form of nonionic CuCO3. In addition, copper has a strong tendency to chelate using available organic matter.
How and in what compounds does copper react with water?
Copper metal is a corrosion-resistant material under normal conditions.
Solubility of copper and/or its compounds in water
Elemental copper metal is insoluble in water, as are copper oxide and copper sulfate. On the other hand, copper(I) chloride has a solubility in water of 200 mg/l, and copper sulfate up to 220 g/l.
How can copper get into water?
Copper is found in various minerals such as chalcopyrite, malachite , azurite or cuprite. Despite possible weathering, it can only be found in small quantities in natural waters. Copper compounds are also used in agriculture and are thus released into the environment. Some copper and its compounds can be recycled. However, they often end up in waste incineration plants, from where, in turn, they can be released into the environment to a certain extent.
Don't underestimate the amount of copper that dissolves when rainwater interacts with roofing materials. The copper content of sewage sludge also often increases as a result.
Solubility of copper in nitric acid
This reaction is possible because the metal is oxidized by a strong reagent. Dilute and concentrated nitric acid exhibits oxidizing properties when dissolving copper.
In the first embodiment, the reaction produces copper nitrate and divalent nitrogen oxide in a ratio of 75% to 25%. The dilute nitric acid process can be described by the following equation:
In the second case, copper nitrate and nitrogen oxides, divalent and tetravalent, are obtained, the ratio of which is 1: 1. This process involves 1 mole of metal and 3 moles of concentrated nitric acid. When copper dissolves, the solution heats up strongly, resulting in thermal decomposition of the oxidizing agent and the release of an additional volume of nitrogen oxides:
The reaction is used in small-scale production related to waste processing or removal of coating from waste. However, this method of dissolving copper has a number of disadvantages associated with the release of large amounts of nitrogen oxides. Special equipment is required to capture or neutralize them. These processes are very expensive.
The dissolution of copper is considered complete when the formation of volatile nitrogen oxides completely ceases. The reaction temperature is between 60 and 70 °C. The next step is to drain the solution from the chemical reactor. There are small pieces of metal at the bottom that have not reacted. Water is added to the resulting liquid and filtered.