05/06/2020 Author: VT-METALL
Issues discussed in the material:
- How to obtain non-ferrous metals
- Types of light non-ferrous metals
- Types of heavy non-ferrous metals
- Types of minor non-ferrous metals
- Types of alloying non-ferrous metals
- Noble non-ferrous metals
- Examples of substances included in the group of non-ferrous metals
- About the types and marking of non-ferrous metal scrap
Various types of non-ferrous metals are widely used in modern industries. They are especially valued where product resistance to use in aggressive environments is required. But how is the division into ferrous and non-ferrous metals made? The latter include those that do not contain iron. There are quite a few groups of such materials, but there is no clear classification.
In our article we will talk about the main categories of non-ferrous metals and talk about the classification of their scrap.
Production of non-ferrous metals
It takes a lot of effort and material resources to extract non-ferrous metals, since they are found in the ground in small quantities, and it is almost impossible to find them in their pure form.
The mined ore is sent to a non-ferrous metals plant for further processing through special technologies, including a number of metallurgical processes, such as:
- Pyrometallurgy. In this case, the metal is obtained and purified at high temperatures. This is how approximately 60% of zinc, all lead, and about 95% of copper are made.
- Hydrometallurgy. The metal is extracted from ore, concentrates and production waste through the use of chemical reagents and subsequent isolation from aqueous solutions.
- Electrometallurgy. The production of metals and their compounds is based on electrolysis, that is, non-ferrous metals are isolated from solutions or melts of their compounds by passing a direct electric current through them. In particular, this technology is used to produce aluminum.
Toxicity
Despite lithium's important biological role in our bodies, it can be dangerous. The lightest metal is quite toxic and can cause poisoning. When burned, it provokes irritation and swelling of the mucous membranes. If a piece of whole metal gets on them, the same thing will happen.
Lithium should not be handled without gloves. By interacting with moisture in the air or moisture on the skin, it easily causes burns. You need to be even more careful with molten metal, as its activity increases significantly. When working with it, you need to remember that it is an alkali. You can reduce its effect on the skin with ordinary vinegar.
In the body, lithium increases the stability of the immune system and improves the functioning of the nervous system. But its excess is accompanied by dizziness, drowsiness, and loss of appetite. Metal poisoning leads to decreased libido, muscle weakness, and weight gain. In this case, vision and memory may deteriorate and coma may occur. When working with lithium, you should always wear gloves, a protective suit and goggles.
Types of light non-ferrous metals
Non-ferrous metals are widely distributed in nature and are characterized by strength, low density and high chemical reactivity. They began to be mined back in the 19th century. They are obtained by electrolysis of salts in molten form, electro- and metallothermy. Many light non-ferrous metals are used to produce alloys.
Below we will consider the types of light non-ferrous metals.
- Aluminum.
Silver-colored metal with low density and high strength, melting point - about +700 ° C. In industry it is used as part of alloys. It can be easily cut, sawed, drilled, welded and bent.
VT-metall offers services:
It is commonly used to form alloys with metals having different properties, such as copper, nickel, magnesium and silicon. Such connections are very durable and resistant to adverse weather conditions. Aluminum has high thermal and electrical conductivity.
We recommend articles on metalworking
- Steel grades: classification and interpretation
- Aluminum grades and areas of their application
- Defects in metal products: causes and search methods
- Magnesium.
The color of the metal is silver-white, there is an oxide film on the surface. Magnesium has a low density, but is easy to process, resistant to flammable substances such as gasoline, kerosene, mineral oils, but soluble in acids. Not magnetic. Characterized by low elasticity and low casting properties. Not resistant to corrosion.
- Titanium.
Non-magnetic light metal of silver color with a blue tint. Has high strength and corrosion resistance. Among the disadvantages are low thermal and electrical conductivity. At temperatures above +400 °C, titanium loses its mechanical properties, and when it reaches +540 °C it becomes brittle.
When combined with metals such as aluminum, manganese, chromium, molybdenum, etc., the mechanical properties of titanium increase. Such alloys differ from each other in strength, depending on the alloying metal. Titanium compounds are widely in demand in shipbuilding, mechanical engineering, and aircraft construction. They are used in the production of rocketry, household appliances, etc.
The lightest and strongest metal
Aluminum
In search of the golden mean between lightness and strength, most chemists agree that aluminum is such a metal.
Aluminum is the golden mean between light and durable metals
Aluminum was discovered in 1825 by the Dane Oersted. The first product made from aluminum was a baby rattle. Since then, the metal, which is unpretentious in processing, has found such wide application that this substance has rightfully received the title of metal of the 20th century. It is used to produce everything without which our modern life cannot be imagined: from building structures to garden tools, fittings and cutlery.
Types of heavy non-ferrous metals
The raw materials for the production of heavy non-ferrous metals are sulfide and oxidized polymetallic ores. In this case, various production techniques and methods can be used, the main task of which is to maximize the extraction of valuable components from ore.
When processing heavy non-ferrous metals, pyro- and hydrometallurgy technologies are used. As a result of these processes, crude metals are formed, which are further refined and used in industry.
- Copper.
A common heavy metal with high electrical and thermal conductivity and ductility.
Copper combines well with other components. Due to this, the resulting alloys are widely used in mechanical engineering.
- Zinc.
Not all alloys containing zinc are used in industrial applications. It's all about its fragility. However, when heated to +150 °C, it can be easily forged and rolled. Zinc is resistant to corrosion, but is destroyed when interacting with acid and alkali.
- Lead.
It has a gray color with a bluish tint. Its melting point is +327 °C. Lead is both a heavy and soft metal; it lends itself well to hammering without hardening. When casting it takes on various shapes. Unaffected by hydrochloric, sulfuric, acetic and nitric acids.
Reactions with lithium
Given its alkaline nature, it can be assumed that it is very active. However, metal is the calmest representative of its group. At normal room temperature, lithium reacts weakly with oxygen and many other substances. It shows its “violent temper” after heating, then it reacts with acids, various gases and bases.
Unlike other alkali metals, it reacts mildly with water, forming hydroxide and hydrogen. There is practically no reaction with dry air. But if it is wet, then lithium slowly reacts with its gases, forming nitride, carbonate and hydroxide.
At certain temperatures, the lightest metal is active with ammonia, ethyl alcohol, halogens, hydrogen, carbon, silicon, and sulfur.
Types of minor non-ferrous metals
Non-ferrous metals also include:
- antimony;
- mercury;
- cadmium.
Antimony is a silvery-white metal with a bluish tint. It is particularly fragile, which allows it to be crushed even with the help of your fingers. However, when combined with other metals, it significantly increases their hardness. Antimony is popular not only in industry, but also in medicine - it is an effective remedy for treating inflammation of the mucous membrane of the eye.
Mercury is a metal in a liquid state. It has been used for a long time in medicine (in thermometers), and is used in modern technologies (in position sensors, ion engines).
Cadmium is a white substance with a metallic tint. Despite its special hardness, it can be easily cut even with a knife. Has properties similar to mercury and zinc. In its pure form, it is poisonous to all living things.
Lithium alloys
The properties of lithium enhance certain qualities of metals, which is why it is often used in alloys. Its reaction with oxides, hydrogen, and sulfides is useful. When heated, it forms insoluble compounds with them, which are easy to extract from molten metals, purifying them of these substances.
To give the alloy corrosion resistance and ductility, it is mixed with magnesium and aluminum. Copper alloyed with it becomes denser and less porous, and conducts electricity better. The lightest metal increases the hardness and ductility of lead. At the same time, it increases the melting point of many substances.
Thanks to lithium, the metal becomes durable and resistant to damage. At the same time, it does not weigh them down. That is why alloys based on it are used in space engineering and aviation. Mixtures with cadmium, copper, scandium and magnesium are mainly used.
Types of alloying non-ferrous metals
These include, for example:
- molybdenum;
- tungsten;
- cobalt;
- vanadium.
Molybdenum does not occur in nature in its pure form. It is inferior in strength to tungsten, but is easier to process. Mainly used in the aviation and missile industries.
Tungsten is one of the most refractory and dense metals, has a silvery-white color, and is similar in appearance to platinum. It is widely used in the production of various cutting tools, jewelry, aircraft and missile parts, and ammunition.
Cobalt has a silvery color with a yellow or blue tint. Alloys with its predominance are used in the manufacture of various medical parts and instruments.
Vanadium is a highly plastic silver-white metal that is actively used in the automotive industry, as it significantly increases the anti-corrosion and mechanical properties of steel.
Lightest artificial metal
Microlattis
It is known that the lightest natural metal is lithium.
However, in 2015, scientists at the University of California demonstrated an ultra-light material similar in strength to metals, but a hundred times lighter than foam. It is 99.99% air. Moreover, the thickness of its walls is only 100 nanometers - a thousand times thinner than a human hair. If you place a piece of microlattis on a dandelion, the flower, whose “cap” can be destroyed even by a slight breeze, does not become deformed.
Microlattis is so light that it does not deform dandelions
The structure of microlattis resembles human bones. The material has the same cellular structure, consisting of hollow nickel tubes intersecting crosswise. Thanks to its structure, the material is able to withstand enormous loads for its weight.
Microlattis under a microscope
Boeing immediately announced its intention to use microlattis for the “aircraft of the future.”
Noble non-ferrous metals
These include:
- gold;
- silver;
- platinum
Gold is a metal with extreme chemical resistance. It cannot be oxidized even in molten form - it dissolves only under the influence of a composition of hydrochloric and nitric acids. It is distinguished by high fluidity rates and is excellent for processing. The price on the exchange of non-ferrous metals for 1 g is 2,450 rubles.
Silver is a metal characterized by ductility, high electrical and thermal conductivity, easy forging, and does not oxidize under the influence of O2.
Types of rare non-ferrous metals
These metals include:
- tantalum;
- niobium.
Tantalum is a hard and dense metal with a silvery hue, but can be easily processed. The main industries of its application are metallurgical, chemical and nuclear.
Niobium is a gray substance with a steely tint. It is distinguished by its special refractoriness and paramagnetic properties. Used in aviation and radio engineering industries.
Finding in nature and meaning
The lightest metal has about 30 minerals of its own, but only 5 of them are used in industry: pentalite, amblygonite, lepidolite, zinnwaldite and spodumene. In addition, it is located in salt lakes. In total, the earth's crust contains 0.005% of this metal.
Large industrial reserves of lithium are found on all continents. It is mined in Brazil, Australia, South Africa, Canada, USA and other countries. After which it is used in electronics, metallurgy, laser materials, nuclear energy and even medicine.
There is a high lithium content in humus, which indicates its participation in the cycle of natural substances. The metal is present in the body of animals, as well as in many plants. Peaches, mushrooms, radishes, potatoes, and carrots are rich in lithium.
In our body it is found in the liver, blood, lungs, bones and other organs. Lack of lithium leads to disturbances in the functioning of the nervous system and brain. It increases the body's resistance to disease and activates the activity of enzymes. It is used to fight Alzheimer's disease, mental disorders, sclerosis, and various addictions.
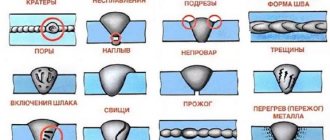
Types and marking of non-ferrous metal scrap
Some types of non-ferrous metal scrap can be recycled for reuse.
The table below presents a list of metals and the number of types of scrap for each of them.
| Metal name | Number of types of scrap |
| Aluminum | 32 |
| Tungsten | 17 |
| Cadmium | 2 |
| Cobalt | 3 |
| Magnesium | 8 |
| Copper | 13 |
| Brass | 23 |
| Bronze | 15 |
| Molybdenum | 9 |
| Lead | 11 |
| Mercury | 6 |
| Tin | 10 |
| Nickel | 26 |
Alloys of non-ferrous metals are most often delivered to recycling collection points. Belonging to a particular type of metal is determined by the element predominant in the alloy in percentage terms. The assessment occurs through the use of special equipment.
Scrap of non-ferrous metals is usually divided into groups depending on:
- origin;
- chemical components;
- physical condition of the material at the time of delivery.
Non-ferrous metal becomes scrap if:
- defect detected;
- written off as industrial waste;
- is substandard;
- became scrap of finished products.
The chemical components of scrap can only be determined in the laboratory, after which it can be correctly said which metal or alloy it belongs to.
The most valuable secondary raw materials are unalloyed metals containing small amounts of impurities. However, the physical parameters of scrap are not as important as the chemical ones.
In accordance with these characteristics, types of non-ferrous metal scrap are usually divided as follows:
- class A – scrap and pieces of waste;
- class B – shavings, wire and small lump waste;
- class B - waste in powder form (not common, usually with certain metals, including tungsten, molybdenum, cobalt and titanium).
- class G – other types of secondary raw materials.
All colored scrap undergoes thorough testing for:
- chemical and radiation pollution;
- degree of explosion hazard.
After it is completed, a certificate is issued confirming the safety of the scrap. Without this document, further transportation is impossible.
The saturation of scrap with harmful substances should not exceed the norm established by GOST 12.1.005.
The Ministry of Natural Resources and Ecology of the Russian Federation distinguishes five classes of non-ferrous scrap metal, representing chemical, radiation and explosion hazards. This is scrap:
- Dangerous for the ecological environment. Such wastes include mercury, as well as plutonium and polonium.
- It is highly dangerous and requires 30 years to eliminate the consequences of its use. It includes alloys of metals such as lead, molybdenum and cobalt.
- It has a moderate danger, after application of which it takes 10 years to restore the environment. It includes scrap with impurities of metals such as copper, iron, zinc, nickel, aluminum and silver.
- It has low danger and requires 3 years to eliminate the consequences of its use. Bronze belongs to this class.
- With a low level of danger that does not harm the ecosystem. This includes most types of non-ferrous metal scrap.
Scrap metal collection points are required to have a license. This need is due to the fact that non-ferrous metal poses a danger to people and the environment. GOST regulates the determination of scrap grades in accordance with designated quality parameters.
In this case, some characteristics inherent in scrap are taken into account, including:
- size;
- origin;
- degree of homogeneity;
- percentage of blockage;
- chemical components;
- physical deterioration;
- dimensions.
A representative sample allows you to identify the quality of scrap. During its transportation, labeling containing the following information is a mandatory requirement:
- Name;
- designation in accordance with GOST;
- type of secondary raw materials;
- alloy grade.
The marking must be securely attached to the cargo during transportation and storage.
To determine the grade of metal, you only need to look at a special document - a stamp book, which contains information about all the markings of the metal or raw material you are interested in.
Lecture 1. General characteristics of metals
Plan.
1. Features of the structure of metal atoms. The position of metals in the PS.
2. The structure of simple substances - metals. Metal bond and metal crystal lattice.
3. Physical properties of metals.
4. Chemical properties of metals.
5. Occurrence in nature and general methods of obtaining metals.
6. The concept of corrosion.
1. The outer electronic level of elements that are classified as metals is filled with electrons less than half (usually 1-2). Usually these are s electrons. Typical metals (i.e. those exhibiting metallic properties to the maximum extent) include s-elements (elements of groups 1 and 2, main subgroups), i.e. those who are just beginning to fill a new layer. Moreover, metallic properties increase with increasing atomic radius (number of electronic layers). Metals also include all d elements (i.e. those in which the d-sublevel of the penultimate layer is filled); they are located in secondary subgroups in all groups. d elements are called transition metals, atypical. Metals also include f-elements (lanthanides and actinides, which are usually located in separate rows at the bottom of the table
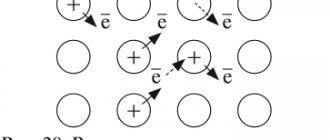
2. Metals have a crystalline, i.e. ordered structure. Some of the atoms in the crystal lattice are ionized, i.e. has lost electrons from the outer electron layer and free electrons are evenly distributed throughout the crystal. They attach to metal ions and they turn into atoms, while other metal atoms at this time lose their electrons and turn into ions. Those. a kind of exchange of electrons occurs. Valence electrons are simultaneously in the possession of all atoms and ions of the metal (i.e., they are attracted to them) and are called “electron gas.” Such a bond between atoms in a metal crystal is called metallic. And the crystal lattice of metals is also called metallic. Metals have a non-molecular structure. Metallic bonding is non-localized between specific atoms.
3. The general physical properties of metals are due to the similarity in the structure of the crystal lattice and the same type of chemical bond.
This bond is strong enough, so metals at no. are in a solid state of aggregation. An exception is mercury Hg with a melting point (-39 ). But the melting temperatures of metals can be different. Eat
low-melting metals (less than 100), for example cesium Cs (28) and other alkali metals, magnesium, aluminum... There are also refractory metals (more than 100), for example tungsten W (3380), as well as chromium, molybdenum, copper, titanium, iron...
The metal crystal lattice is dense and therefore all metals are opaque and reflect the incident light (white color, metallic luster). The exceptions are gold and copper, which absorb part of the spectrum and have a yellow color.
All metals can conduct electricity. This property is due to the presence of mobile electrons in the crystal lattice. Under normal conditions, the metals of the copper subgroup have the highest electrical conductivity: Ag, Cu, Au and aluminum. These metals are used as conductors in electrical engineering and radio electronics. Tungsten, nickel, and chromium have high resistance. Heating elements of electrical appliances are made from them.
Metals conduct not only electric current, but also heat. Metals that conduct electricity well have high thermal conductivity. This is also due to the ability of electrons to move and transfer thermal energy.
All metals are ductile to one degree or another (that is, they are irreversibly deformed under mechanical loads) and can be forged. Gold is the most ductile; it can be used to produce a thread 500 times thinner than a human hair, i.e. practically invisible. Alkali metals are also soft. Chromium and tungsten are considered very hard metals. And antimony is so brittle at room temperature that it can be ground into powder. Plastic deformation is explained by the fact that in metals the chemical bonds in the metal crystal lattice do not break; the ions and atoms simply move relative to each other.
All metals are insoluble in water, but dissolve in each other. Such solutions are called alloys.
Based on their density, metals are divided into light (less than 5 g/cm3) and heavy. Light metals include alkali and alkaline earth metals, titanium, and aluminum. Heavy metals include zinc, iron, copper, mercury, lead, and gold. The heaviest is osmium (22.6 g/cm3).
4. The general chemical properties of metals are also determined by the general features in their structure. They all complete the outer electron layer by donating valence electrons. Consequently, in chemical reactions, simple substances - metals - are always reducing agents.
Me0 – ne- → Me+n For example:
The more easily atoms give up electrons, the stronger the reducing agent the metal is. But we must remember that metal ions are capable of accepting electrons, i.e. exhibit oxidizing ability. Moreover, the easier an atom loses electrons, the worse the corresponding ion accepts them. Those. for example, sodium is an active reducing agent, but the sodium ion does not exhibit oxidizing activity. The low-active copper atom is reluctant to lose its electrons, and the copper ion is a fairly strong oxidizing agent.
Various substances can act as oxidizing agents for metals, but in nature and technology, the most important are gaseous oxygen and the hydrogen ion, which is present in water and acid solutions. Look carefully at the table.
| Li | K | Ca | Na | Mg | Al | Mn | Zn | Cr | Fe | Ni | Sn | Pb | H2 | Cu | Hg | Ag | Pt | Au | |
| Reducing ability of metals in a free state | Increasing | ||||||||||||||||||
| Interaction with atmospheric oxygen | Oxidizes quickly at normal temperatures | Oxidizes slowly at normal temperatures or when heated | Do not oxidize | ||||||||||||||||
| Interaction with water | At normal temperature, H2 is released and hydroxide is formed | When heated, hydrogen is released and oxides are formed | Does not displace hydrogen from water | ||||||||||||||||
| Interaction with acids | Displaces hydrogen from dilute acids (except HNO3) | Does not displace hydrogen from dilute acids | |||||||||||||||||
| React with HNO3 and conc. H2SO4 | Dissolves only in aqua regia | ||||||||||||||||||
| Being in nature | Only in connections | In connections and in free form | Mainly in free form | ||||||||||||||||
| Methods of obtaining | Electrolysis of melts | Reduction with coal, CO, active metals, electrolysis of aqueous solutions | |||||||||||||||||
| Oxidizing power of metal ions | Li | K | Ca | Na | Mg | Al3 | Mn | Zn | Cr | Fe | Ni | Sn | Pb | H | Cu | H Hg | Ag | Pt | Au |
| Increasing |
Metal oxidation: Zn + O2 → ZnO
Interaction of active metals with water: K + H2O → KOH + H2
Interaction of metals with acids: Mn + HCl → MnCl2+ H2
Interaction of metals with other oxidizing agents: Fe + CuSO4 → FeSO4 + Cu
5. Those metals that can be oxidized by hydrogen ions from natural water (usually acidified by interaction with various acidic oxides) or free air oxygen, i.e. cannot exist in the form of a simple substance in nature. This means that the “noble metals” gold, silver and platinum are usually found in a free state. Some low-active metals can also be found, but such deposits are rare and currently have no economic significance. But this was precisely the reason why the first metals known to mankind were copper, mercury, lead, tin... Base metals are found in nature in the form of compounds. For active metals these are salts: chlorides, sulfates, phosphates, carbonates. Moreover, the lower the solubility of these compounds, the greater the likelihood of encountering them. Less active metals occur as oxides or as sulfides. Moreover, before iron there are mainly oxides, and after it there are mainly sulfides.
Naturally, the methods for obtaining metals also depend on their activity. Obtaining precious metals usually involves separating them from waste rock. There are many methods for this; they are described in fiction and specialized literature. The production of metals from their compounds can be called in one word: “reduction”. Those. The chemical essence of these processes is to force metal ions to accept electrons. What can be used as a reducing agent? What substances easily give up their electrons? That's right, metals! This means that active metals can be used to obtain less active metals from their oxides. For example:
Mg + SnO2 → MgO + Sn
Such methods of obtaining metals, depending on the reducing agent, are called magnesium thermia, calcium thermia, sodium thermia...
Hydrogen is also a good reducing agent:
H2 + Cr2O3 → H2O+ Cr
But these reducing agents (both hydrogen and active metals) have a significant drawback - high cost. After all, they are not found in nature in a free form, and their production is expensive. Therefore, such reducing agents are used only if it is economically justified, i.e. for obtaining rare and expensive metals. And metals that need to be obtained in very large quantities (iron) are reduced with a cheaper reducing agent - carbon. It is used in the form of coke, and previously charcoal was used.
С + Fe2O3→ СO2 + Fe
And if the metal is found in nature not in the form of an oxide, but in the form of a sulfide, then the ore is first roasted, and only then the resulting oxide is reduced. For example:
NiS + O2→ NiO + SO2
How are the most active metals restored? Where can I find such a strong reducing agent? Such an active reducing agent will be electric current. The process is called electrolysis. The metal oxide or its chloride is melted and an electric current is passed through the melt. For example:
At the cathode the process of metal reduction occurs: Na+ + e- → Na0
The anion oxidation process occurs at the anode: Cl- - e- → Cl20
The overall reaction looks like this: NaCl → Cl20 + Na0
Plan.
1. Features of the structure of metal atoms. The position of metals in the PS.
2. The structure of simple substances - metals. Metal bond and metal crystal lattice.
3. Physical properties of metals.
4. Chemical properties of metals.
5. Occurrence in nature and general methods of obtaining metals.
6. The concept of corrosion.
1. The outer electronic level of elements that are classified as metals is filled with electrons less than half (usually 1-2). Usually these are s electrons. Typical metals (i.e. those exhibiting metallic properties to the maximum extent) include s-elements (elements of groups 1 and 2, main subgroups), i.e. those who are just beginning to fill a new layer. Moreover, metallic properties increase with increasing atomic radius (number of electronic layers). Metals also include all d elements (i.e. those in which the d-sublevel of the penultimate layer is filled); they are located in secondary subgroups in all groups. d elements are called transition metals, atypical. Metals also include f-elements (lanthanides and actinides, which are usually located in separate rows at the bottom of the table
2. Metals have a crystalline, i.e. ordered structure. Some of the atoms in the crystal lattice are ionized, i.e. has lost electrons from the outer electron layer and free electrons are evenly distributed throughout the crystal. They attach to metal ions and they turn into atoms, while other metal atoms at this time lose their electrons and turn into ions. Those. a kind of exchange of electrons occurs. Valence electrons are simultaneously in the possession of all atoms and ions of the metal (i.e., they are attracted to them) and are called “electron gas.” Such a bond between atoms in a metal crystal is called metallic. And the crystal lattice of metals is also called metallic. Metals have a non-molecular structure. Metallic bonding is non-localized between specific atoms.
3. The general physical properties of metals are due to the similarity in the structure of the crystal lattice and the same type of chemical bond.
This bond is strong enough, so metals at no. are in a solid state of aggregation. An exception is mercury Hg with a melting point (-39 ). But the melting temperatures of metals can be different. Eat
low-melting metals (less than 100), for example cesium Cs (28) and other alkali metals, magnesium, aluminum... There are also refractory metals (more than 100), for example tungsten W (3380), as well as chromium, molybdenum, copper, titanium, iron...
The metal crystal lattice is dense and therefore all metals are opaque and reflect the incident light (white color, metallic luster). The exceptions are gold and copper, which absorb part of the spectrum and have a yellow color.
All metals can conduct electricity. This property is due to the presence of mobile electrons in the crystal lattice. Under normal conditions, the metals of the copper subgroup have the highest electrical conductivity: Ag, Cu, Au and aluminum. These metals are used as conductors in electrical engineering and radio electronics. Tungsten, nickel, and chromium have high resistance. Heating elements of electrical appliances are made from them.
Metals conduct not only electric current, but also heat. Metals that conduct electricity well have high thermal conductivity. This is also due to the ability of electrons to move and transfer thermal energy.
All metals are ductile to one degree or another (that is, they are irreversibly deformed under mechanical loads) and can be forged. Gold is the most ductile; it can be used to produce a thread 500 times thinner than a human hair, i.e. practically invisible. Alkali metals are also soft. Chromium and tungsten are considered very hard metals. And antimony is so brittle at room temperature that it can be ground into powder. Plastic deformation is explained by the fact that in metals the chemical bonds in the metal crystal lattice do not break; the ions and atoms simply move relative to each other.
All metals are insoluble in water, but dissolve in each other. Such solutions are called alloys.
Based on their density, metals are divided into light (less than 5 g/cm3) and heavy. Light metals include alkali and alkaline earth metals, titanium, and aluminum. Heavy metals include zinc, iron, copper, mercury, lead, and gold. The heaviest is osmium (22.6 g/cm3).
4. The general chemical properties of metals are also determined by the general features in their structure. They all complete the outer electron layer by donating valence electrons. Consequently, in chemical reactions, simple substances - metals - are always reducing agents.
Me0 – ne- → Me+n For example:
The more easily atoms give up electrons, the stronger the reducing agent the metal is. But we must remember that metal ions are capable of accepting electrons, i.e. exhibit oxidizing ability. Moreover, the easier an atom loses electrons, the worse the corresponding ion accepts them. Those. for example, sodium is an active reducing agent, but the sodium ion does not exhibit oxidizing activity. The low-active copper atom is reluctant to lose its electrons, and the copper ion is a fairly strong oxidizing agent.
Various substances can act as oxidizing agents for metals, but in nature and technology, the most important are gaseous oxygen and the hydrogen ion, which is present in water and acid solutions. Look carefully at the table.
| Li | K | Ca | Na | Mg | Al | Mn | Zn | Cr | Fe | Ni | Sn | Pb | H2 | Cu | Hg | Ag | Pt | Au | |
| Reducing ability of metals in a free state | Increasing | ||||||||||||||||||
| Interaction with atmospheric oxygen | Oxidizes quickly at normal temperatures | Oxidizes slowly at normal temperatures or when heated | Do not oxidize | ||||||||||||||||
| Interaction with water | At normal temperature, H2 is released and hydroxide is formed | When heated, hydrogen is released and oxides are formed | Does not displace hydrogen from water | ||||||||||||||||
| Interaction with acids | Displaces hydrogen from dilute acids (except HNO3) | Does not displace hydrogen from dilute acids | |||||||||||||||||
| React with HNO3 and conc. H2SO4 | Dissolves only in aqua regia | ||||||||||||||||||
| Being in nature | Only in connections | In connections and in free form | Mainly in free form | ||||||||||||||||
| Methods of obtaining | Electrolysis of melts | Reduction with coal, CO, active metals, electrolysis of aqueous solutions | |||||||||||||||||
| Oxidizing power of metal ions | Li | K | Ca | Na | Mg | Al3 | Mn | Zn | Cr | Fe | Ni | Sn | Pb | H | Cu | H Hg | Ag | Pt | Au |
| Increasing |
Metal oxidation: Zn + O2 → ZnO
Interaction of active metals with water: K + H2O → KOH + H2
Interaction of metals with acids: Mn + HCl → MnCl2+ H2
Interaction of metals with other oxidizing agents: Fe + CuSO4 → FeSO4 + Cu
5. Those metals that can be oxidized by hydrogen ions from natural water (usually acidified by interaction with various acidic oxides) or free air oxygen, i.e. cannot exist in the form of a simple substance in nature. This means that the “noble metals” gold, silver and platinum are usually found in a free state. Some low-active metals can also be found, but such deposits are rare and currently have no economic significance. But this was precisely the reason why the first metals known to mankind were copper, mercury, lead, tin... Base metals are found in nature in the form of compounds. For active metals these are salts: chlorides, sulfates, phosphates, carbonates. Moreover, the lower the solubility of these compounds, the greater the likelihood of encountering them. Less active metals occur as oxides or as sulfides. Moreover, before iron there are mainly oxides, and after it there are mainly sulfides.
Naturally, the methods for obtaining metals also depend on their activity. Obtaining precious metals usually involves separating them from waste rock. There are many methods for this; they are described in fiction and specialized literature. The production of metals from their compounds can be called in one word: “reduction”. Those. The chemical essence of these processes is to force metal ions to accept electrons. What can be used as a reducing agent? What substances easily give up their electrons? That's right, metals! This means that active metals can be used to obtain less active metals from their oxides. For example:
Mg + SnO2 → MgO + Sn
Such methods of obtaining metals, depending on the reducing agent, are called magnesium thermia, calcium thermia, sodium thermia...
Hydrogen is also a good reducing agent:
H2 + Cr2O3 → H2O+ Cr
But these reducing agents (both hydrogen and active metals) have a significant drawback - high cost. After all, they are not found in nature in a free form, and their production is expensive. Therefore, such reducing agents are used only if it is economically justified, i.e. for obtaining rare and expensive metals. And metals that need to be obtained in very large quantities (iron) are reduced with a cheaper reducing agent - carbon. It is used in the form of coke, and previously charcoal was used.
С + Fe2O3→ СO2 + Fe
And if the metal is found in nature not in the form of an oxide, but in the form of a sulfide, then the ore is first roasted, and only then the resulting oxide is reduced. For example:
NiS + O2→ NiO + SO2
How are the most active metals restored? Where can I find such a strong reducing agent? Such an active reducing agent will be electric current. The process is called electrolysis. The metal oxide or its chloride is melted and an electric current is passed through the melt. For example:
At the cathode the process of metal reduction occurs: Na+ + e- → Na0
The anion oxidation process occurs at the anode: Cl- - e- → Cl20
The overall reaction looks like this: NaCl → Cl20 + Na0