Iron ore is obtained in the usual way: open-pit or underground mining and subsequent transportation to initial preparation, where the material is crushed, washed and processed.
The ore is poured into a blast furnace and blasted with hot air and heat, which turns it into molten iron. It is then removed from the bottom of the furnace into molds known as pigs, where it cools to produce cast iron. It is turned into wrought iron or processed into steel in several ways.
What is steel?
In the beginning there was iron. It is one of the most common metals in the earth's crust. It can be found almost everywhere, combined with many other elements, in the form of ore. In Europe, the beginning of working with iron dates back to 1700 BC.
In 1786, French scientists Berthollet, Monge and Vandermonde accurately determined that the difference between iron, cast iron and steel was due to different carbon content. However, steel, made from iron, quickly became the most important metal of the Industrial Revolution. At the beginning of the 20th century, global steel production was 28 million tons, six times more than in 1880. By the beginning of the First World War, its production was 85 million tons. Within a few decades, it practically replaced iron.
Carbon content affects the characteristics of the metal. There are two main types of steel: alloyed and unalloyed. Steel alloy refers to chemical elements other than carbon added to iron. Thus, to create stainless steel, an alloy of 17% chromium and 8% nickel is used.
There are currently more than 3,000 cataloged brands (chemical compounds), not counting those created to meet individual needs. All of them contribute to making steel the most suitable material for solving the challenges of the future.
The third stage of production - steel deoxidation
The reduction of iron oxide, which is dissolved in the liquid metal, occurs.
An increase in the oxygen content in the metal during melting is necessary for the oxidation of impurities, but in finished steel, oxygen is a harmful impurity because it reduces the mechanical properties of the steel. Steel deoxidation is carried out by two methods: diffusion and precipitation.
Diffusion deoxidation occurs due to slag deoxidation. In crushed form, ferrosilicon, ferromanganese and aluminum are transferred to the surface of the slag. These deoxidizers restore iron oxide and at the same time reduce its content in the slag. This means that iron oxide, which is dissolved in steel, goes into this slag. The oxides that are formed during this process remain in the slag, and the iron, already in a reduced form, goes into steel, and the content of non-metallic inclusions in it decreases and its quality increases.
Precipitation deoxidation occurs due to the introduction into liquid steel of soluble deoxidizers (ferrosilicon, ferromanganese, aluminum), which contain elements with a higher affinity for oxygen compared to iron. In the end, after deoxidation, iron is reduced and oxides are created: SiO2, MnO, Al2O5, which have a lower density compared to steel, and are discharged into slag.
Depending on the level of deoxidation, the following types of steel can be smelted: - boiling - not completely deoxidized in the furnace. Deoxidation of such steel continues in the mold during solidification of the ingot, due to the interaction of carbon and iron oxide: FeO + C = Fe + CO.
The carbon monoxide that has formed is removed from the steel, ensuring the removal of hydrogen and nitrogen from the steel, gases are removed in the form of bubbles, causing it to boil. Boiling steel does not have non-metallic inclusions, and therefore has a high degree of ductility.
- calm - obtained with absolute deoxidation in the ladle and in the furnace.
- semi-quiet - characterized by intermediate deoxidation between boiling and calm steels. It is partially deoxidized in the ladle and in the furnace, and partially in the mold, due to the interaction of carbon and iron oxide contained in the steel.
Alloying of steel occurs by introducing pure metals or ferroalloys in a certain amount into the melt. Alloying elements that have a lower affinity for oxygen than iron (Co, Ni, Cu, Mo) do not oxidize during casting and smelting, and therefore are introduced at some time during smelting. Alloying elements that have a greater affinity for oxygen than iron (Mn, Si, Cr, Al, Ti, V) are introduced into the metal after deoxidation or together with it at the final stage of smelting, and sometimes into the ladle.
Raw materials for steelmaking: primary and secondary
Smelting this metal using many components is the most common mining method. Charge materials can be either primary or secondary. The main composition of the charge is usually 55% pig iron and 45% remaining scrap metal. Ferroalloys, converted cast iron and technically pure metals are used as the main element of the alloy; secondary elements, as a rule, include all types of ferrous metal.
Iron ore is the most important and basic raw material in iron and steel industry. To produce a ton of cast iron, about 1.5 tons of this material are required. To produce one ton of pig iron, about 450 tons of coke are used. Many metallurgical plants even use charcoal.
Water is an important raw material for the iron and steel industry. It is mainly used for coke quenching, blast furnace cooling, coal furnace door steam production, hydraulic equipment operation and waste water disposal. It takes about 4 tons of air to produce a ton of steel. Flux is used in a blast furnace to remove impurities from smelting ore. Limestone and dolomite combine with the extracted impurities to form slag.
Both blast and steel furnaces are lined with refractories. They are used for liner furnaces designed for smelting iron ore. Silica or sand is used for molding. Non-ferrous metals are used to produce various grades of steel: aluminum, chromium, cobalt, copper, lead, manganese, molybdenum, nickel, tin, tungsten, zinc, vanadium, etc. Among all these ferroalloys, manganese is widely used in steel smelting.
Iron waste obtained from dismantled plant structures, machinery, old vehicles, etc. is recycled and widely used in this industry.
Mining
There are several methods for extracting ore. The one that is found most economically feasible is used.
- Open method of development - or quarry. Designed for shallow mineral rock. For mining, a quarry is dug to a depth of up to 500 m and a width depending on the thickness of the deposit. Iron ore is extracted from the quarry and transported by vehicles designed to carry heavy loads. As a rule, this is how high-grade ore is mined, so there is no need to enrich it.
- Mine - when the rock occurs at a depth of 600–900 m, shafts are drilled. Such development is much more dangerous because it involves underground blasting: the discovered layers are blasted, and then the collected ore is transported upward. Despite its dangers, this method is considered more effective.
- Hydraulic mining - in this case, wells are drilled to a certain depth. Pipes are lowered into the mine and water is supplied under very high pressure. The water jet crushes the rock, and then the iron ore is lifted to the surface. Borehole hydraulic production is not widespread, as it requires high costs.
Next, the technology and iron manufacturing processes are discussed.
Cast iron for steel
Steel smelting using cast iron is carried out much more often than with other materials. Cast iron is a term that usually refers to gray iron, but it is also identified with a large group of ferroalloys. Carbon makes up about 2.1 to 4 wt.%, while silicon typically makes up 1 to 3 wt.% in the alloy.
Iron and steel are smelted at a melting point between 1150 and 1200 degrees, which is about 300 degrees lower than the melting point of pure iron. Cast iron also exhibits good fluidity, excellent machinability, and resistance to deformation, oxidation, and casting.
Steel is also an alloy of iron with variable carbon content. The carbon content of steel ranges from 0.2 to 2.1 mass%, and it is the most economical alloying material for iron. Smelting steel from cast iron is useful for a variety of engineering and structural purposes.
Steel smelting using a single-slag process
If the steel is not subject to strict requirements for sulfur content, and sometimes in the case of subsequent processing of steel in a ladle with synthetic slag, smelting can be carried out under one slag. Dephosphorization of the metal is combined with melting. The oxidation slag is not specifically removed from the furnace, and after achieving the required carbon content and ≤0.035% P, ferrosilicon and ferromanganese, as well as the necessary alloying elements, are introduced into the bath. The metal is finally deoxidized in a ladle with lump ferrosilicon and aluminum in an amount of 0.5-1.0 kg/t.
The duration of smelting in a single-slag process is reduced by 1-1.5 hours, energy consumption is reduced by 15-20%, the consumption of slag-forming and ferroalloys is reduced, and at the same time the labor intensity of smelting is reduced.
Iron Ore for Steel
The process of steel smelting begins with the processing of iron ore. The rock containing the iron ore is crushed. The ore is mined using magnetic rollers. Fine-grained iron ore is processed into coarse-grained lumps for use in the blast furnace. Coal is purified of impurities in a coke oven, resulting in a nearly pure form of carbon. The mixture of iron ore and coal is then heated to produce molten iron or pig iron, which is used to make steel.
In the main oxygen furnace, molten iron ore is the main raw material and is mixed with varying amounts of scrap steel and alloys to produce different grades of steel. An electric arc furnace melts recycled steel scrap directly into new steel. About 12% of steel is made from recycled material.
Process Features
The steel production process occurs sequentially in three stages.
The first stage is the melting of the rock. At the stage of its implementation, a melt is formed in the bath and the metal is oxidized, simultaneously giving oxygen to silicon, phosphorus and manganese.
One of the main tasks of this stage is phosphorus removal. Its implementation requires a relatively low temperature and the presence of a sufficient amount of FeO. When the ingredients interact, phosphorus anhydride forms an unstable compound (FeO)3 + P2O5 with iron oxide.
The presence of the more stable base CaO in the slag causes the replacement of FeO. As a result, it binds phosphorus anhydrite into another compound (CaO)4 x P2O5 + 4 Fe, which is what was required to be achieved.
Pure Fe was released in the melt, and phosphorus formed slag, which is removed from the metal surface and disposed of as unnecessary. Since phosphorus anhydride transforms the composition of the slag, the process must be continuous.
Therefore, FeO must be continuously replenished by loading new batches of iron ore and scale, which introduce ferrous slag into the melt.
Smelting technology
Melting is the process by which a metal is obtained either as an element or as a simple compound from its ore by heating above its melting point, usually in the presence of oxidizing agents such as air or reducing agents such as coke.
In steelmaking technology, a metal that combines with oxygen, such as iron oxide, is heated to a high temperature, and the oxide is formed in combination with the carbon in the fuel, which comes out as carbon monoxide or carbon dioxide. Other impurities, collectively called veins, are removed by the addition of a stream with which they combine to form slag.
Modern steel melting uses a reverberatory furnace. Concentrated ore and stream (usually limestone) are loaded into the top, and molten matte (a compound of copper, iron, sulfur and slag) is pulled from the bottom. A second heat treatment in a converter oven is necessary to remove iron from the matte surface.
Process Features
Together with them, slag-forming substances are loaded: lime and bauxite. The body is surrounded by a support ring attached to the pivot pins. With their help, the vessel is tilted and the finished steel is poured through this hole - the tap hole. Bottom blowing is carried out through through holes (tuyeres) made in the bottom of the furnace.
Historically, it has been the custom that the method used everywhere is called the Thomas or Bessemer method. In the last century, the open-hearth process became predominant. The regenerator is heated by purging furnace gases, after which it heats the cold air entering the melt.
In modern designs, the top method is more often used, in which blowing at high speed is carried out through nozzles lowered to the metal surface. In Russia, it is primarily the top blowing of furnaces that is used.
Being under a stream of air, cast iron intensively oxidizes in the contact zone. Since its concentration is much higher than other impurities, iron oxide is predominantly formed. But it dissolves in the slag. Therefore, the metal is enriched with the released oxygen.
C, Cr and Mn are oxidized, reducing the percentage in the metal structure. Oxidation is accompanied by the release of heat. Due to the presence of slags CaO and FeO before heating, phosphorus is removed at the very beginning of blowing.
The slag is merged with it and a new one is created. Steel production is accompanied by express analyzes and monitoring of current changes using control devices installed in the furnace. The phosphorus content in cast iron should not exceed 0.075%.
Oxygen-convection method
The BOF process is the leading steelmaking process in the world. World production of converter steel in 2003 amounted to 964.8 million tons or 63.3% of total production. Converter production is a source of environmental pollution. The main challenges of this are the reduction of emissions, discharges and waste reduction. Their essence lies in the use of secondary energy and material resources.
Exothermic heat is generated by oxidation reactions during blowdown.
The main process of steelmaking using our own reserves:
- Molten pig iron (sometimes called hot metal) from a blast furnace is poured into a large fireproof lined container called a ladle.
- The metal in the ladle is sent directly to the main steel production or pre-processing stage.
- High-purity oxygen at a pressure of 700-1000 kilopascals is injected at supersonic speeds onto the surface of the iron bath through a water-cooled lance that is suspended in a vessel and held several feet above the bath.
The decision to pretreat depends on the quality of the hot metal and the desired final steel quality. The very first converters with removable bottoms, which could be detached and repaired, are still in use. The spears used for blowing have been changed. To prevent jamming of the tuyere during purging, slotted cuffs with a long tapering copper tip were used. The tip tips, after combustion, burn off the CO produced by blowing into CO2 and provide additional heat. Darts, refractory balls and slag detectors are used to remove slag.
Oxygen-converter method of steel production
Steel production today is carried out mainly in this way. BOF production recently accounted for up to 60% of global steel production.
However, this percentage is decreasing due to the advent of electric arc furnaces (EAFs). The furnaces are purged with pure oxygen (99.5%) under high pressure.
The product of an oxygen converter furnace is steel with specified chemical properties. It enters a continuous casting machine (CCM), where the material solidifies into a bloom or slab. To obtain certain stringent parameters, the metal is recycled.
Oxygen-convection method: advantages and disadvantages
Does not require costs for gas purification equipment, since dust formation, i.e. evaporation of iron, is reduced by 3 times. Due to a decrease in the yield of iron, an increase in the yield of liquid steel is observed by 1.5 - 2.5%. Another advantage is that the intensity of purging in this method increases, which makes it possible to increase the productivity of the converter by 18%. The quality of steel is higher because the temperature in the blowing zone is reduced, which leads to a decrease in the formation of nitrogen.
The disadvantages of this method of steel smelting have led to a decrease in demand for consumption, since the level of oxygen consumption increases by 7% due to the high consumption of fuel combustion. There is an increased hydrogen content in the processed metal, which is why it is necessary to carry out purging with oxygen for some time after the end of the process. Among all the methods, the oxygen-converter method has the highest slag formation; the reason is the inability to monitor the oxidation process inside the equipment.
Features of the second stage
The second stage of steel production technology is called steel boiling. The main purpose is to reduce the carbon content by percentage due to oxidation. FeO + C = CO + Fe.
The oxidation reaction occurs more intensely during boiling and is accompanied by heat absorption. Therefore, it is necessary to create a constant flow of heat into the bath, as well as to equalize the temperature in the melt.
During this oxidation reaction, carbon monoxide gas CO is intensively released, which causes rapid boiling in the liquid aggregate state, for this reason the process is called boiling. To ensure that excess carbon is more intensively converted into oxide, the production of high-quality steel involves injecting pure oxygen and adding scale to the molten structure. This is why the quality of raw materials for steel production is so important. All source materials undergo scrupulous testing.
It is important at this stage to remove sulfur, which improves the quality of the final steel. The sulfur used in the components is not present in direct form, but in the form of iron sulfide FeS.
At high temperatures, the component also reacts with CaO oxide, forming calcium sulfide CaS, which dissolves in the slag without combining with iron. This allows the sulfide to be easily removed outside the bath.
Converter steel production
Open hearth method
The open hearth process comprised the bulk of processing of all steel produced in the world for most of the 20th century. William Siemens in the 1860s sought a means of increasing the temperature in a metallurgical furnace, resurrecting an old proposal to use the waste heat generated by the furnace. He heated the brick to a high temperature, then used the same path to introduce air into the kiln. Preheated air significantly increased the flame temperature.
Natural gas or atomized heavy oils are used as fuel; air and fuel are heated before combustion. The furnace is loaded with liquid blast iron and steel scrap along with iron ore, limestone, dolomite and fluxes.
The stove itself is made of highly refractory materials, such as magnesite bricks for the hearths. Open hearth furnaces weigh up to 600 tons and are typically installed in groups so that the massive auxiliary equipment required to charge the furnaces and process the liquid steel can be efficiently utilized.
Although the open hearth process has been almost completely replaced in most industrialized countries by the basic oxygen process and electric arc furnace, it produces about 1/6 of all steel produced worldwide.
Lecture on Materials Science “Steel Production” (SPO)
- Steel production
Become
– iron-carbon alloys containing almost 1.5% carbon; with a higher content, the hardness and brittleness of steels significantly increase and they are not widely used.
The main source materials for steel production are cast iron and steel scrap (scrap).
The content of carbon and impurities in steel is significantly lower than in cast iron. Therefore, the essence of any metallurgical processing of cast iron into steel is to reduce the content of carbon and impurities by selectively oxidizing them and converting them into slag and gases during the smelting process.
Iron is oxidized primarily when cast iron reacts with oxygen in steelmaking furnaces:
.
Simultaneously with iron, silicon, phosphorus, manganese and carbon are oxidized. The resulting iron oxide at high temperatures gives up its oxygen to more active impurities in the cast iron, oxidizing them.
Steel smelting processes are carried out in three stages.
The first stage is melting the charge and heating the liquid metal bath. The most important task of the stage is phosphorus removal.
To remove phosphorus, low temperatures of the metal and slag bath and sufficient content in the slag are required. To increase the content in the slag and accelerate the oxidation of impurities, iron ore and scale are added to the furnace, creating ferrous slag . The second stage - boiling of the metal bath - begins as it warms up to higher temperatures.
As the temperature rises, the carbon oxidation reaction occurs more intensely, occurring with the absorption of heat:
.
To oxidize carbon, a small amount of ore, scale, or oxygen is injected into the metal.
The third stage, steel deoxidation, involves the reduction of iron oxide dissolved in the liquid metal.
Steel is deoxidized in two ways: precipitation and diffusion
.
Precipitation deoxidation is carried out by introducing into liquid steel soluble deoxidizers (ferromanganese, ferrosilicon, aluminum) containing elements that have a greater affinity for oxygen than iron.
Alloying of steel is carried out by introducing ferroalloys or pure metals in the required quantity into the melt. Alloying elements, which have a lower affinity for oxygen than iron (), do not oxidize during melting and casting, so they are introduced at any time during melting. Alloying elements, which have a greater affinity for oxygen than iron (), are introduced into the metal after deoxidation or simultaneously with it at the end of the melt, and sometimes into the ladle.
- Steel production in open hearth furnaces
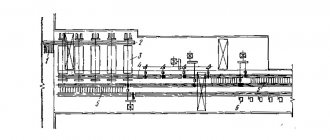
Martin process (1864-1865, France). Until the seventies it was the main method of steel production. The method is characterized by relatively low productivity and the possibility of using secondary metal – steel scrap. The capacity of the furnace is 200…900 tons. The method makes it possible to produce high-quality steel.
The open hearth furnace (Fig. 2.2.) according to its design and principle of operation is a flame reverberatory regenerative furnace. Gaseous fuel or fuel oil is burned in the smelting space. The high temperature for obtaining steel in a molten state is provided by heat recovery from furnace gases.
A modern open-hearth furnace is a horizontally elongated chamber made of refractory brick. The working melting space is limited from below by the hearth 12, from above by the arch 11 ,
and on the sides there are 5 front and 10 rear walls. The hearth has the shape of a bathtub with slopes towards the walls of the furnace. In the front wall there are loading windows 4 for supplying charge and flux, and in the rear wall there is a hole 9 for releasing finished steel.
Rice. Scheme of an open hearth furnace
A characteristic of the working space is the area of the furnace bottom, which is calculated at the level of the thresholds of the loading windows. At both ends of the melting space there are furnace heads 2, which serve to mix fuel with air and supply this mixture into the melting space. Natural gas and fuel oil are used as fuel.
To heat air and gas when operating on low-calorie gas, the furnace has two regenerators 1.
Regenerator - a chamber in which a nozzle is placed - a refractory brick laid out in a cage, designed to heat air and gases.
The gases leaving the furnace have a temperature of 1500...1600 0C. Entering the regenerator, the gases heat the nozzle to a temperature of 1250 0C. Air is supplied through one of the regenerators, which, passing through the nozzle, is heated to 1200 0C and enters the furnace head, where it is mixed with fuel; at the outlet of the head, a torch 7 is formed, directed at the charge 6.
The exhaust gases pass through the opposite head (left), cleaning devices (slag tanks), which serve to separate slag and dust particles from the gas and are sent to the second regenerator.
Cooled gases leave the furnace through chimney 8.
After cooling, the nozzles of the right regenerator switch the valves, and the flow of gases in the furnace changes direction.
The temperature of the flame reaches 1800 0C. The torch heats the furnace working space and the charge. The torch promotes the oxidation of charge impurities during smelting.
The duration of melting is 3...6 hours, for large furnaces - up to 12 hours. The finished melt is released through a hole located in the rear wall at the lower level of the hearth. The hole is tightly plugged with low-caking refractory materials, which are knocked out when the melt is released. The furnaces operate continuously until they are stopped for major repairs - 400...600 heats.
The main technical and economic indicators of steel production in open hearth furnaces are:
- furnace productivity - steel removal from 1 m2 of hearth area per day (t/m2 per day), on average is 10 t/m2; R
- Fuel consumption per 1 ton of steel produced averages 80 kg/t.
As furnaces become larger, their economic efficiency increases.
3. Steel production in oxygen converters
The oxygen-converter process is the smelting of steel from liquid cast iron in a converter with a main lining and blowing oxygen through a water-cooled lance.
The first experiments in 1933-1934 - Mozgovoy.
On an industrial scale - in 1952-1953 at factories in Linz and Donawitz (Austria) - it was called the LD process. Currently, the method is the main one in the mass production of steel.
An oxygen converter is a pear-shaped vessel made of steel sheet, lined with base brick.
Converter capacity is 130...350 tons of liquid cast iron. During operation, the converter can be rotated 360° to load scrap, pour cast iron, drain steel and slag.
The charge materials of the oxygen-converter process are liquid pig iron, steel scrap (no more than 30%), lime for slag removal, iron ore, as well as bauxite and fluorspar for slag liquefaction.
The sequence of technological operations when melting steel in oxygen converters is presented in Fig. 2.3.
Rice. The sequence of technological operations when melting steel in oxygen converters
After the next steel melting, the outlet hole is sealed with a refractory mass and the lining is inspected and repaired.
Before melting, the converter is tilted and scrap rice is loaded using charging machines. (2.3.a), cast iron is poured at a temperature of 1250...1400 0C (Fig. 2.3.b).
After this, the converter is turned to the working position (Fig. 2.3.c), a cooled lance is inserted inside and oxygen is supplied through it at a pressure of 0.9...1.4 MPa. Simultaneously with the start of blowing, lime, bauxite, and iron ore are loaded. Oxygen penetrates the metal, causing it to circulate in the converter and mix with the slag. A temperature of 2400 0C develops under the tuyere. In the zone of contact of the oxygen jet with the metal, iron is oxidized. Iron oxide dissolves in the slag and metal, enriching the metal with oxygen.
Phosphorus is removed at the beginning of purging the bath with oxygen, when its temperature is low (the phosphorus content in cast iron should not exceed 0.15%). If the phosphorus content is high, to remove it, it is necessary to drain the slag and introduce a new one, which reduces the productivity of the converter.
Sulfur is removed throughout the entire melting process (the sulfur content in cast iron should be up to 0.07%).
In oxygen converters, steels with different carbon contents, boiling and calm, as well as low-alloy steels are smelted. Alloying elements in molten form are introduced into the ladle before steel is released into it.
Melting in converters with a capacity of 130...300 tons ends in 25...30 minutes.
- Steel production in electric furnaces
Electric melting furnaces have advantages compared to other melting units:
a) it is easy to regulate the thermal process by changing current parameters;
b) it is possible to obtain a high temperature of the metal,
c) the ability to create an oxidizing, reducing, neutral atmosphere and vacuum, which allows the metal to be deoxidized with the formation of a minimum amount of non-metallic inclusions.
Electric furnaces are used for smelting structural, high-alloy, tool, and special alloys and steels.
There are arc and induction electric furnaces.
5. Arc melting furnace
The arc furnace diagram is shown in Fig.
Rice. Arc melting furnace diagram
The arc furnace is powered by three-phase alternating current. Has three cylindrical electrodes 9
from a graphitized mass, fixed in electrode holders
8
, to which electric current is supplied via cables
7
.
An electric arc occurs
between the electrode and the metal charge 3 The furnace body has the shape of a cylinder. Outside it is enclosed in a durable steel casing 4
, inside it is lined with basic or acid brick
1
.
The melting space is limited by walls 5
, hearth
12
and roof
6.
The removable roof
6
has holes for electrodes.
In the wall of the housing there is a working window 10
(for draining slag, loading ferroalloys, taking samples), closed during melting with a shutter.
The finished steel is discharged through a drain hole with a drain chute 2
.
The furnace rests on sectors and has a drive 11
for tilting towards the working window or chute. The furnace is loaded with the roof removed.
The capacity of the furnaces is 0.5…400 tons. In metallurgical shops, electric furnaces with a basic lining are used, and in foundries - with an acid lining.
6. Induction crucible melting furnaces
They smelt the highest quality corrosion-resistant, heat-resistant and other steels and alloys.
Capacity from tens of kilograms to 30 tons.
The diagram of an induction crucible furnace is shown in Fig. 3.2.
Rice. Diagram of an induction crucible furnace
The furnace consists of a water-cooled inductor 3
, inside of which there is a crucible
4
(basic or acidic refractory materials) with a metal charge, a single-phase alternating current of high frequency (500...2000 Hz) passes through the inductor from a high-frequency generator.
When passing current through an inductor in metal 1
located in the crucible, powerful eddy currents are induced, which ensures heating and melting of the metal. To reduce heat loss, the stove has a removable roof
2
.
The crucible is made from acidic (quartzite) or basic (magnesite powder) refractories. To release the melt, the furnace is tilted towards the drain chute.
Under the influence of the electromagnetic field of the inductor during melting, intensive circulation of liquid metal occurs, which helps to accelerate chemical reactions, obtain a metal with a homogeneous chemical composition, quickly float up non-metallic inclusions, and equalize the temperature.
In induction furnaces, steel and alloys are smelted from alloy waste by
remelting
, or from pure charge iron and scrap with the addition of ferroalloys
by fusion
.
7. Steel casting
From the melting furnaces, steel is released into a ladle, which is carried by an overhead crane to the steel casting site. From the ladle, the steel is poured into the molds or molds of a continuous casting machine. In molds or crystallizers, steel hardens and ingots are obtained, which are rolled and forged.
Molds
– cast iron molds for the production of ingots.
Molds are made with square, rectangular, round and multifaceted cross sections.
Ingots with a square cross-section are converted into long products: I-beams, channels, angles. Rectangular ingots - into sheets. Round ingots are used to make pipes and wheels. Ingots with a multifaceted cross-section are used for the manufacture of forgings.
Calm and boiling carbon steels are poured into ingots weighing up to 25 tons, alloy and high-quality steels into ingots weighing 0.5...7 tons, and some types of high-alloy steels into ingots weighing up to several kilograms.
Steel is poured into molds from above (Fig. 3.3.a), from below (by siphon) (Fig. 3.3.b) and on continuous casting machines (Fig. 3.4).
Rice. Pouring steel into molds
a – from above; b – from below (siphon)
Steel is poured into the molds from above directly from the ladle. 1
. This eliminates the consumption of metal on the gates and simplifies the preparation of equipment for casting. The disadvantages include the poorer quality of the surface of the ingots, due to the presence of oxide films from metal splashes that harden on the walls of the mold.
Advantages and disadvantages of this method
The advantages include ease of use and ease of production of alloy steel with various additives that give the material various specialized properties. The necessary additives and alloys are added immediately before the end of smelting.
The disadvantages include reduced efficiency compared to the oxygen-converter method. Also, the quality of steel is lower compared to other methods of metal smelting.
Production of non-ferrous metals
Copper production.
Copper is obtained mainly by pyrometallurgical methods. Pyrometallurgy is a set of metallurgical processes occurring at high temperatures. The production of copper from copper ores includes their beneficiation, roasting, smelting into an intermediate product - matte , smelting blister copper from the matte (converting) and its purification from impurities ( refining ).
For the production of copper, copper ores containing 1–6% Cu, as well as copper waste and its alloys are used.
Blister copper contains 98.4 – 99.4% Cu and a small amount of impurities. This copper is poured into molds. Blister copper is refined to remove harmful impurities and gases.
After fire refining, copper with a purity of 99 - 99.5% is obtained (Fig. 21). Pigs are cast from it for smelting copper alloys (bronze and brass) or plates for electrolytic refining. Electrolytic refining is carried out to obtain pure copper from impurities (more than 99.5% Cu).
Rice. 21. Production of refined copper
Aluminum production.
The main method of aluminum production currently is electrolytic. Electrolysis is a set of electrochemical oxidation-reduction processes that occur on electrodes immersed in an electrolyte during the passage of an electric current.
The main raw materials for aluminum production are aluminum ores: bauxite , nepheline, alunite, kaolin.
Aluminum production includes:
- obtaining anhydrous, impurity-free aluminum oxide (Al2O3 alumina). Alumina is obtained from bauxite by treating it with alkali;
- obtaining cryolite from fluorspar 2H3AlF6;
- electrolysis of alumina in molten cryolite;
During the electrolysis process, aluminum collects at the bottom of the bath under a layer of electrolyte. It is periodically removed using a special device. For normal operation of the bath, a little aluminum is left at the bottom (Fig. 22.
Aluminum produced by electrolysis is called raw aluminum. It contains metallic and non-metallic impurities and gases. Impurities are removed by refining, for which chlorine is blown through molten aluminum. Then the liquid aluminum is kept in a ladle or in an electric furnace for 30 - 45 minutes at a temperature of 690 - 730 ° C to float non-metallic inclusions and release gases from the metal. After refining, the purity of primary aluminum is 99.5 – 99.85%. In Fig. 23. photograph of the Ural Aluminum Plant.
Rice. 22. Aluminum production
Rice. 23. Ural Aluminum Plant
Magnesium production.
For the production of magnesium, the electrolytic method is most widespread (Fig. 24).
Rice. 24. Magnesium production scheme
The main raw materials for the production of magnesium are carnallite, magnesite, dolomite, and bischofite.
Magnesium production includes:
- obtaining pure anhydrous magnesium salts (magnesium chloride MgCl2);
- electrolysis of these salts in a molten state, obtaining rough magnesium which contains 5% impurities;
- refining of crude magnesium, i.e. melt it with fluxes at a temperature of 700...750°C and mix. Non-metallic impurities turn into slag. Then the furnace is cooled to a temperature of 670°C, and magnesium is poured into molds into pigs.
Titanium production.
Titanium is produced by the magnesium-thermal method. Titanium production includes:
- enrichment of titanium ores;
- smelting titanium slag from them with subsequent production of titanium tetrachloride from it;
- recovery from the last metal titanium with magnesium.
The raw material for titanium production is titanomagnetite ores, from which ilmenite concentrate (TiO2, FeO, Fe2O3 and waste rock) is isolated. This concentrate received its name from the presence of the mineral ilmenite FeO... TiO2.
Ilmenite concentrate is smelted in a mixture with charcoal and anthracite, where iron and titanium oxides are reduced. The resulting titanium slag is subjected to chlorination in special furnaces. Next, the resulting titanium tetrachloride is mixed with pig magnesium in reactors (Fig. 25) at a temperature of 950 - 1000 ° C and its reduction occurs. The result is a porous mass - a sponge.
Titanium sponge is melted using the VAR method. The vacuum in the furnace protects titanium from oxidation and helps clean it from impurities. The resulting titanium ingots have defects, so they are re-melted and used as consumable electrodes. As a result, the purity of titanium is 99.6 – 99.7%. After secondary remelting, the ingots (Fig. 26) are used for pressure treatment. In Fig. Figure 27 shows a product made from titanium.
Rice. 25. Reactors for the reduction of titanium tetrachloride
Rice. 26. Titanium ingots
Rice. 27 Product made of titanium
408
Electric steelmaking method
The modern method of smelting steel using its own reserves is a furnace that heats charged material using an electric arc. Industrial arc furnaces range in size from small units with a load capacity of about one ton (used in foundries to produce cast iron products) to 400 ton units used in secondary metallurgy.
Arc furnaces used in research laboratories may have a capacity of only a few tens of grams. Industrial electric arc furnace temperatures can be up to 1800 °C (3.272 °F), while laboratory installations can exceed 3000 °C (5432 °F).
Arc furnaces differ from induction furnaces in that the charging material is directly exposed to the electric arc, and the current in the terminals passes through the charged material. The electric arc furnace is used for steel production, consists of a refractory lining, usually water-cooled, is large in size, and covered with a retractable roof.
The oven is mainly divided into three sections:
- Shell consisting of side walls and a lower steel bowl.
- The hearth consists of a refractory that extends the lower bowl.
- The fire-lined or water-cooled roof can be designed as a ball section or as a truncated cone (conical section).
Steel production methods
There are several methods for producing steel, each with its own specific advantages and disadvantages. The chosen method determines what properties the material can be obtained with. Main methods of steel production:
- Martin's method. This technology involves the use of special furnaces that are capable of heating raw materials to a temperature of about 2000 degrees Celsius. Considering the methods for producing alloy steels, we note that this method also allows the addition of various impurities, due to which steels of unusual composition are obtained. The open hearth method is based on the use of special furnaces.
- Electric steel melting method. In order to obtain high quality material, steel is produced in electric furnaces. By using electrical energy to heat raw materials, it is possible to precisely control the progress of the oxidation process and the release of slags. In this case, it is important to ensure the appearance of toxins. They are a transmitter of oxygen and heat. This technology allows you to reduce the concentration of harmful substances, for example, phosphorus and sulfur. Electric smelting can take place in a wide variety of environments: excess pressure, vacuum, or a certain atmosphere. Conducted research indicates that electric steel is of the highest quality. Technology is used to produce high-quality high-alloy, corrosion-resistant, heat-resistant and other types of steel. To convert electrical energy into heat, a cylindrical arc furnace with a spherical bottom is used. To ensure the most favorable melting conditions, the internal space is finished using heat-resistant metal. The device can only operate when connected to a three-phase network. It is worth considering that the electrical supply network must withstand a significant load. The source of thermal energy is an electric arc that occurs between the electrode and the molten metal. Temperatures can be over 2000 degrees Celsius.
- Oxygen converter. Continuous casting of steel in this case is accompanied by active injection of oxygen, due to which the oxidation process is significantly accelerated. This manufacturing method is also used to produce cast iron. It is believed that this technology has the greatest versatility and allows the production of metals with various properties.
The methods for producing galvanized steel are not very different from those considered. This is due to the fact that the change in the qualities of the surface layer occurs through chemical-thermal treatment.
There are other steel production technologies that are highly efficient. For example, methods based on the use of vacuum induction furnaces, as well as plasma arc welding.
Advantages and disadvantages of the method
This method occupies a leading position in the field of steel production. The steel smelting method is used to create a high-quality metal that is either completely devoid of or contains small amounts of unwanted impurities such as sulfur, phosphorus and oxygen.
The main advantage of the method is the use of electricity for heating, thanks to which you can easily control the melting temperature and achieve incredible heating rates for the metal. Automated work will be a pleasant addition to the excellent opportunity for high-quality processing of various scrap metal.
The disadvantages include high energy consumption.
History of steel production
Everyone knows that almost three quarters of the periodic table of chemical elements D.I. Mendeleev, are metals. The place of metals in the modern world is one of the central ones, and their importance for modern man can hardly be overestimated. It would seem that a person knows everything about metals, there are no secrets left for him in this area, but allow us, employees of a company that has been successfully engaged in metal rolling for a long time, to doubt this and introduce you to some secrets from the history of human use of metals. Let's look into the mysterious depths of human history, because it was there that a still young tribe of people became acquainted with metals, discovered some of their magical properties, and learned how to make them useful. However, when exactly this happened and how exactly is the biggest secret and the most important mystery of metals, which it is trying in vain to unravel.
According to legend, the first iron came to people from heaven. It was contained in meteorites. This is confirmed by the words denoting iron in different languages - in ancient Egyptian iron is called “vaaepere”, translated as “born in heaven”, and in ancient Coptic it is called “stone of heaven”. However, the rarity of iron meteorites in nature is confusing, which significantly reduces the likelihood of them being found by ancient people. Scientists are inclined to the terrestrial origin of iron, which confirms the extremely rare occurrence of nuggets in nature.
The oldest metal products were found on the site of settlements that existed about eight thousand years ago! At first, man simply found some metals that occur in nature in a natural, or native state - gold, silver, copper. They sparkled mysteriously, pleasing to the eye, and therefore they were used to make jewelry. However, people soon used native copper as a material for various tools: fishhooks, arrowheads and spears.
How did man begin to extract metal from stone? How did ore mining first begin? Oh, this did not happen right away, and not without the help of divine forces, which in this case were represented by fire. The ancient deities protected people, but they themselves needed protection. To prevent the fire from going out, it was surrounded by stones, and among these stones there were also pieces of copper ore. Under the influence of the magical powers of fire, the ore melted and turned into copper. For a long time the ancient man did not notice these magical transformations, but finally he noticed and began to specially load copper ore into the fire in order to obtain metal. Copper smelted from ore turned out to be stronger than native copper, although it was still inferior in strength to stone - it was too soft. The alloy of copper and tin, bronze, turned out to be much stronger. Bronze tools gradually replaced similar copper ones.
For a long time, iron was valued on a par with gold because it was just as scarce. But in the end, man discovered a relatively cheap production of iron - smelting it from ore in metallurgical furnaces. The Iron Age began on earth, which continues to this day.
And now let's turn to another mystery: when did man learn why metals are produced? Yes, man first learned how metals were made, but for a long time after that he could not understand why. Man could not understand all the transformations of iron: sometimes it turned out to be hard, but brittle, and sometimes, on the contrary, too soft, but tools made from it bend, flatten and quickly become dull. Thus, the history of ore mining is the history of various experiments that were carried out with metals and continued until the last quarter of the 19th century. It was then that the Russian scientist P.P. Anosov scientifically substantiated the production of steel. It took him 10 years to do this.
In our 21st century, steel is produced at specially equipped metallurgical plants. Where iron ore is first smelted in huge blast furnaces, where it is turned into pig iron. Cast iron, in turn, is melted, but in open hearths, convectors or electric furnaces, and then it turns into steel. Specialists of various profiles “conjure” this magical transformation: sinterers, metallurgical engineers, converters, roasters, smelters, casters, slingers, who easily operate various metallurgical units.
How did you get steel when you didn’t have all this arsenal of smart machines? In the East, as well as in Egypt, the British Isles, Ancient Hellas, and Ancient Rus', steel was smelted from carefully prepared iron ore in small clay vessels (crucibles). Iron ore was first crushed into small pieces, then these pieces were burned over a fire. In the process, sulfur, phosphorus and other substances were burned out, which, when found in the ore, worsen the properties of the metal. The ancient craftsmen, of course, had no idea about the existence of all these substances and their effect on metal; they simply knew, based on experience, that better steel was made from crushed and fired ore.
After completion of roasting, the ore was poured into the crucible, and covered in layers with charcoal powder; As a rule, 10-12 layers were made (coal layer - ore layer - coal layer). Coal in that case played the role of a heat carrier, as it burned and melted the ore. To make the combustion more intense, there was a hole at the base of the crucible into which air was pumped through large leather bellows. Thus, the highest temperature was created in the crucible, under the influence of which the ore melted, and the carbon, which makes up coal, removed oxygen from the ore, and it turned into iron. Metal-SK is doing just that.
Later, clay crucibles were replaced by small furnaces, which produced more metal. However, in the East for a very long time they remained faithful to precisely that method of creating steel, which required the use of a crucible. Maybe that’s why for an eastern master, getting iron is not the end result. The end result was damask steel, known and revered throughout the world, for no other could compare with it in hardness and yet flexibility. The secret of making damask steel was passed down from father to son and has not been preserved for certain. But it is known that after receiving the iron, the master took out miraculous plants from secluded corners (the ancient masters were sure that plant juices, having strength, flexibility, and viscosity, transferred these properties to the metal) and threw them into the hole of the crucible, but most importantly - in the proportion which was known only to him. And so, the plants burned, truly transferring their magical properties to the iron, turning it into steel. It was certainly possible to establish that, along with the roots and leaves, the craftsmen added graphite powder to the metal, of course, only in certain proportions. And the wise masters did not know that it was graphite, which they considered rather an auxiliary material, that turned iron into steel. The fact is that graphite is pure carbon, which plays one of the main roles in metal production. The first most important rule of metallurgy is that only that alloy is considered steel in which the amount of carbon does not exceed two percent. The second most important rule is that the more carbon, the stronger the steel, but the less ductile, and vice versa.
This is how, until the middle of the last century, by selecting the exact amount of carbon, the difficult task of combining two opposites in metal - strength and ductility - was solved. So, the decisive benefits of graphite have been proven. But what about flowers and roots? What is their use? The fact is that they contain a huge amount of different inorganic substances: iron, molybdenum, vanadium. These substances influenced steel in different ways, giving it special unique properties. Speaking about the ancient production of steel, one cannot fail to touch upon such an important point as its hardening. This is the most mysterious, most exciting moment of making a special kind of steel. Hardening was invented in Ancient Egypt, where craftsmen, wanting to quickly cool a forged product, immersed it in very cold water, and as a result noted that after this procedure the metal became much stronger.
The ancient people mistakenly believed that hardening directly depended on the qualities of the liquid into which the hot metal was immersed. But this fallacy gave rise to many fantastic, sophisticated experiments. Thus, in Baghdad, metal was cooled by thrusting it into the muscular body of a slave, who was supposed to transfer his strength to the weapon. In the Middle Ages, there was a known recipe for hardening steel, the main ingredient in which was the urine of a red-haired boy. Tell me, dark superstitions? And you'll be right. Simply, blades are actually better tempered in blood or urine than in simple well water, because this process should ideally take place slowly, which is what happens in salt solutions. Or if the blade is cooled in the wind, as steel was tempered in ancient Damascus.
What then to do with modern methods? What is their appeal? In the privacy of scientific knowledge over mythopoetic knowledge, which distinguishes modern metallurgy from ancient metallurgy, but does not at all exclude its beauty. This is confirmed by well-coordinated, precise work, where every detail made of metal is calculated to the smallest detail and is beautiful in its impeccability. Yes, in the modern world a lot is decided by mathematical formulas, numerical dependencies, and precise calculations. So, even on paper, you can predict in advance the properties that the resulting steel will have, having previously calculated the entire technology for its production. That is why the modern metal industry amazes with a huge range of steels: ultra-strong, wear-resistant, heat-resistant, acid-resistant. This approach was called compositional, and metallurgists called it composers. But it’s true that if the famous aphorism calls architecture “music frozen in stone,” then many metal products cannot be called anything other than music frozen in metal.