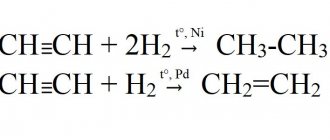Hydrocarbons containing a triple bond are called alkynes. Alkynes are often referred to as acetylenes, named after the first member of the homologous series. They form a homologous series with the general formula CnH2n-2. According to the IUPAC nomenclature, the names of alkynes are formed by replacing the ending “-ane” in the name of the corresponding alkane with the ending “-in”:
The monovalent radicals corresponding to alkynes are called alkynyl
,
propargyl
.
HC≡C–CH2–
In the series of acetylenes, structural isomerism is observed in the position of the triple bond in the carbon chain and isomerism of alkyl radicals associated with alkynyl.
Pentin-2
In industry, acetylene is used very widely and a method for its production by thermolysis of methane has been developed.
or hydrogenation of carbon at 3000 °C:
The reaction is carried out using an electric arc between carbon electrodes in a flow of hydrogen, because The heating time must be very short to avoid the reverse process of acetylene decomposition into elements.
There is also a carbide method for producing some acetylenes:
This reaction is actually the hydrolysis of acetylenides, which are essentially metal carbides.
Among the laboratory methods for obtaining acetylenes, one can note a variety of elimination reactions, among which the dehydrohalogenation of vic-
dihalides
It should be borne in mind that in an alkaline environment, alkynes are prone to rearrangements with migration of the triple bond:
Therefore, it is recommended to use sodium amide in liquid ammonia for elimination. Under these conditions, mainly terminal acetylenides are formed, and heme-acetylenides
dihalides:
Other synthesis methods involve the transformation of some alkynes into others; they will be discussed below.
The carbon atoms in acetylene are sp-hybridized and are connected to each other by one s- and two p-bonds. Therefore, the acetylene molecule is linear (bond angle 180°). The CºC bond length is 121 pm (for comparison - in ethane 154 pm, in ethylene 134 pm), the C-H bond length is 106 pm (in ethane 110 pm, in ethylene 107 pm).
The energy of a triple carbon-carbon bond is 833 kJ/mol, which is less than the total energy for three σ bonds (339x3=1017 kJ/mol) and a combination of one σ and two π bonds (339 + 2 272 = 883 kJ/mol). This can be seen as a result of the mutual repulsion of the bonding electrons of the three bonds, which are forced to be close together in space. An acetylene molecule can be imagined as a cylinder formed by orbitals of π-bonds, from the ends of which C-H σ-bonds emerge. The hydrogen atoms have a shorter bond to the C atoms than in alkenes and alkanes because the carbon atom is in the sp
most electronegative.
The reason for the shortening of the C-H bond in alkynes compared to alkenes and alkanes is that the sp
orbital, having a larger contribution of the s-character (50%), initially lies lower in energy than
sp 2
- (33.3%) and
sp 3
– (75%) orbitals. This leads to an increase in the strength of the C-H bonds in acetylenes (the energy of homolytic dissociation is 502 kJ/mol) relative to the strength of the C-H bonds in alkanes and alkenes (414 kJ/mol and 439 kJ/mol, respectively). The acetylene proton is largely deshielded, since the electron pair is shifted towards the carbon atom to a greater extent than towards those in the sp 2 and sp 3 hybrid states. The sp carbon atom can be considered to be more electronegative than carbon in other valence states.
The two degenerate HOMOs of acetylene (E = -1088.6 kJ/mol) lie lower than the HOMOs of ethylene (E = -963 kJ/mol).
Based on these ideas, the main properties of acetylenes can be explained:
– electrophilic and nucleophilic addition reactions are characteristic of acetylenes;
– terminal alkynes are characterized by increased C-H acidity (higher than in alkanes and alkenes).
The main industrial method for producing acetylene is electro- or thermal cracking of methane, pyrolysis of natural gas and the carbide method.
Carbide method (industrial method)
By calcining a mixture of calcium oxide and coke in electric furnaces at 1800–2000°C, calcium carbide is obtained:
When the resulting carbide is exposed to water, calcium hydroxide and acetylene are formed:
Pyrolysis of hydrocarbons (industrial method)
The essence of the method is to pass a mixture of natural gas and air over a special fireproof nozzle, which, when burned, raises the temperature to 1500 °C. Then methane pyrolysis occurs on the nozzle:
Read also: Cjx2 2508 contactor wiring diagram
Natural gas cracking (industrial method)
The method involves passing methane between two metal electrodes at high speed. Temperature 1500-1600°C. From a chemical point of view, the method is similar to the pyrolysis method, differing only in technological and hardware design.
This method uses partial oxidation of methane by using the heat generated during its combustion:
Direct synthesis method
Carbon reacts directly with hydrogen at very high temperatures:
This method is of purely historical significance (production of acetylene in 1863 by M. Berthelot).
Electrolysis of salts of unsaturated carboxylic acids
In 1864, Kekule obtained acetylene by electrolysis of sodium fumarate and sodium maleate:
Acetylene is produced similarly from sodium acrylate.
This method is of purely historical significance.
Dehydrohalogenation of haloalkanes and haloalkenes (laboratory method)
The dehydrohalogenation reaction is carried out by the action of a strong base on dihaloalkanes:
It is convenient to use sodium amide in liquid ammonia as a dehydrohalogenating agent:
Obtaining Acetylene
In the laboratory, acetylene is produced by the action of water on calcium carbide.
CaC2+ 2 H2O = C2H2↑ + Ca(OH)2
as well as during the dehydrogenation of two methane molecules at temperatures above 1400 °C:
Didn't find what you were looking for? Use the search:
Best sayings:
Learn to learn without studying!
10080 – | 7747 – or read all.
91.146.8.87 © studopedia.ru Not the author of the materials posted. But it provides free use. Is there a copyright violation? Write to us | Feedback.
Disable adBlock! and refresh the page (F5)
very necessary
A colorless gas, slightly soluble in water, somewhat lighter than atmospheric air, belonging to the class of alkynes and representing unsaturated carbon is called acetylene. In its structure, all atoms have a triple bond with each other. This substance boils at a temperature of -830 °C. The formula of acetylene indicates that it contains only carbon and hydrogen.
Acetylene is a dangerous substance that can explode if handled carelessly. That is why specially equipped containers are used to store this substance. The gas burns when combined with oxygen, and the temperature can reach 3150 °C.
Acetylene production
Acetylene can be produced in laboratory and industrial conditions. To obtain acetylene in the laboratory, it is enough to drop a small amount of water onto calcium carbide (this is its formula - CaC2). after this, a violent reaction of acetylene release begins. To slow it down, it is permissible to use table salt (NaCl formula).
In an industrial environment, things are somewhat more complicated. To produce acetylene, pyrolysis of methane, as well as propane and butane, is used. In the latter case, the acetylene formula will contain a large number of impurities.
The carbide method of acetylene production ensures the production of clean gas. But, this method of obtaining a product must be provided with a large amount of electricity.
Pyrolysis does not require a large amount of electricity, the whole point is that to produce gas, it is necessary to heat the reactor and for this they use gas circulating in the primary circuit of the reactor. But in the flow that moves there, the gas concentration is quite small.
Isolating acetylene with a pure formula in the second case is not the easiest task and its solution is quite expensive. There are several ways to produce the acetylene formula in an industrial setting.
Electric cracking
The conversion of methane into acetylene occurs in an electric arc furnace, where it is heated to a temperature of 2000-3000 °C. In this case, the voltage on the electrodes reaches 1 kV. Methane is heated to 1600 °C. To obtain one ton of acetylene, it is necessary to spend 13,000 kWh. This is a significant disadvantage of producing acetylene formula.
Technological diagram of cracking
Oxidative pyrolysis
This method is based on mixing methane and oxygen. After producing the mixture, part of it is sent for combustion and the resulting heat is sent to heat the raw materials to a temperature of 16,000 °C. This process is characterized by continuity and rather modest consumption of electrical energy. Today, this method can most often be found in acetylene production plants.
Technological diagram of the oxidative pyrolysis process
In addition to the listed technologies for the production of acetylene formulas, such as homogeneous pyrolysis and low-temperature plasma are used. All of them differ in the amount of energy costs and, as a result, different characteristics of the resulting gas and its formula.
Read also: DIY computer as an oscilloscope
Acetylene cylinders
Structural and molecular formula: acetylene
Gas cylinders, incl. acetylene. Click to enlarge.
Acetylene can be liquefied and solidified, however, both in the gaseous state at pressures above about 7 bar, and in the liquid and solid states, acetylene is shock sensitive and explosive. Therefore, acetylene is always supplied to users in cylinders dissolved in acetone or dimethylformamide and completely filled with a porous filler Agamassan (or AGA-massan, which stands for “AGA composition” in Swedish. AGA is the name of a Swedish manufacturer and supplier of industrial gases , now a division of Linde Gas, founded at one time by the inventor of Agamassan, Gustaf Dahlen. Agamassan's composition includes asbestos, cement, coal and kieselguhr). As an alternative to Agamassan, filler based on kieselguhr or ceramics/lime silicate can be used.
The excess pressure in acetylene cylinders is usually no more than 17 bar, and the outlet pressure from the cylinder is no more than 1 bar, and usually about 0.5 bar.
Acetylene cylinders are usually equipped with both conventional safety valves that operate when the pressure rises, including passing and isothermal, and special safety valves that operate when the temperature rises above 100°C, releasing acetylene into the atmosphere. Such valves act like fusible links.
In Russia, acetylene cylinders are painted white, with the red inscription “Acetylene”.
Advantages
The mention of gas welding immediately brings to mind acetylene. Indeed, this gas is most often used for this process. It, in combination with oxygen, provides the highest combustion temperature of the flame. But in recent years, due to the development of various types of welding, the use of this type of metal joining has decreased somewhat. Moreover, in some industries there has been a complete abandonment of the use of these technologies. But for certain types of repair work it still remains indispensable.
The use of acetylene allows you to obtain the following advantages:
- maximum flame temperature;
- there is the possibility of generating acetylene directly at the workplace or purchasing it in special containers;
- Quite low cost compared to other flammable gases.
However, acetylene also has certain disadvantages that limit its use. The most important thing is the danger of explosion. When working with this gas, it is necessary to strictly observe safety precautions. In particular, work should be carried out in a well-ventilated area. If operating conditions are violated, some defects may appear, for example, burnouts.
Application of acetylene
Acetylene is used in all processes of gas-flame processing of metals (gas welding and gas cutting), due to the high flame temperature, which cannot be achieved when using other combustibles.
For soldering, cutting, surfacing, flame hardening, metallization, gas press welding, welding of non-ferrous metals and alloys, acetylene substitute gases are successfully used:
- propane-butane mixtures
- city gas
- natural gases
- hydrogen
- gasoline vapor
- kerosene vapor
- MAF
- and etc.
In terms of chemical composition, all of them, with the exception of hydrogen, are either compounds or mixtures of various hydrocarbons.
The correct selection and use of substitute gases allows you to achieve high quality welding and cutting, and when gas cutting metals of small thickness, it gives a higher cutting cleanliness.
Gas welding is possible provided that the flame temperature is twice the melting point of the metal being welded. Therefore, substitute gases whose flame temperature is lower than that of acetylene are used for welding metals with a melting point lower than that of steels
For gas cutting, the choice of flammable gas is based on its calorific value, but it must be taken into account that the gas, when burned in a mixture with oxygen, must form a flame with a temperature of at least 2000°C.
The influence of impurities in acetylene on the quality of the weld
Let's look at some more features of the use of acetylene in gas welding - the influence of impurities on the quality of the weld. The following impurities have a harmful effect:
- hydrogen sulfide
- hydrogen phosphide
The above impurities are necessarily removed from acetylene, not only because of the effect on the quality of the weld, but also because of the detrimental effect on the respiratory system and vision of the welder (see the article Explosion hazard, toxicity and self-ignition of acetylene).
Hydrogen sulfide , when burned, forms sulfuric acid, which, when transferred to the weld metal, causes red brittleness. It has been established that the presence of hydrogen sulfide up to 0.007% does not have a harmful effect on the strength of the weld.
Determining the presence of hydrogen sulfide in acetylene is quite easy; you need to hold filter paper soaked in a solution of mercury chloride under a stream of acetylene. In the presence of hydrogen sulfide, the paper will turn white.
The process of purifying hydrogen sulfide is also quite simple - you need to pass acetylene through water, as a result of which hydrogen sulfide will dissolve in water.
Phosphorous hydrogen, when burned, forms phosphoric acid, which, when transferred to the weld metal, causes cold brittleness. It has been established that the presence of hydrogen phosphide up to 0.027% does not have a harmful effect on the strength of the weld.
To determine the presence of hydrogen phosphide, you need to place a piece of filter paper soaked in a ten percent solution of silver nitrate under a stream of acetylene. With a content of hydrogen phosphide of 0.01%, the paper takes on a distinct light yellow color; with a content of more than 0.02%, the paper darkens.
Chemical purification of acetylene from hydrogen phosphide is carried out by passing it through a special cleaning mass - geratol. Geratol is a yellow mass, which, as a result of interaction with hydrogen phosphide, becomes green.
Application of acetylene in the chemical industry
In addition to gas-flame processing, acetylene is used in the chemical industry as the main starting material for the production of a number of important products of organic synthesis: synthetic rubber, plastics, solvents, acetic acid, etc. Next, we will look at how acetylene is used to obtain certain chemical compounds .
Acetaldehyde
The product of the addition of water to acetylene is acetaldehyde. This synthesis was first carried out by M. G. Kucherov in 1881. The reaction proceeds according to the equation:
HC = CH + H2O? CH3 - CHO
The reaction is carried out by passing acetylene through a sulfate solution of mercuric oxide salt at a temperature of 70-80°C.
The use of this reaction was the beginning of the industrial synthesis of organic substances using acetylene as the starting product.
Acetone
When passing a mixture of acetylene and water vapor in a ratio of approximately 1:10 at a temperature of 430-450°C over a zinc-vanadium catalyst, acetone is formed according to the equation:
2C2H2 + 3H2O ? CH3-CO-CH3 + CO2 + H2O
This process has found application on an industrial scale.
Vinyl chloride
When acetylene reacts with hydrogen chloride at 200°C over a catalyst consisting of mercury dichloride supported on activated carbon, vinyl chloride is formed according to the equation:
C2H2 + HCl ? CH2 = CHCl
Vinyl acetate
With acetic acid, also in the presence of mercury salts, acetylene forms vinyl acetate:
C2H3 + CH3COOH ? CH2 = CH-OCO-CH3
Vinyl chloride and vinyl acetate are widely used in the production of plastics.
Acetylene formula
The structure of the acetylene molecule
Acetylene has a simple formula - C2H2. The relatively cheap method of producing it by mixing water and calcium carbide has made it the most widely used gas for joining metals. The temperature at which a mixture of oxygen and acetylene burns causes solid carbon particles to be released.
Acetylene can be delivered to the work site in special containers (gas cylinders), or it can be obtained directly at the workplace using a specially designed reactor. Where the mixing of water and calcium carbide occurs.
Nomenclature
Alkynes and acetylene hydrocarbons are hydrocarbons whose molecules contain at least two carbon atoms that are in a state of sp-hybridization and connected to each other by three bonds.
Alkynes form a homologous series with the general formula CnH2n-2.
The first member of the homologous series is acetylene, which has the molecular formula C2H2 and the structural formula CHCH. Due to the peculiarity of sp-hybridization, the acetylene molecule has a linear structure. The presence of two π-bonds located in two mutually perpendicular planes implies the location of the α-atoms of the substituent groups on the line of intersection of the planes in which the π-bonds are located. Therefore, the bonds of carbon atoms spent on connection with other atoms or groups are rigidly located on a line at an angle of 1800 to each other. The structure of the triple bond system in alkyne molecules is determined by their linear structure.
The structural feature of alkyne molecules suggests the existence of isomerism in the position of the triple bond. Structural isomerism, due to the structure of the carbon skeleton, begins with the fifth member of the homologous series.
1. Isomerism of the triple bond position. For example:
2. Structural isomers. For example:
The first member of the homologous series has the trivial name “acetylene”.
According to rational nomenclature, acetylene hydrocarbons are considered as acetylene derivatives. For example:
According to the IUPAC nomenclature, the names of alkynes are formed by replacing the suffix “an” with “in”. The main chain is chosen so that it contains a triple bond. The numbering of carbon atoms begins from the end of the chain to which the triple bond is closest. If there are double and triple bonds in a molecule, the double bond has a lower number. For example:
The triple bond can be terminal (terminal, such as in propyne) or "internal", such as in 4-methyl-2-pentine.
When naming, the radical -CCH is called “ethynyl”.
Chemical and physical properties
Some chemical properties
The properties of acetylene are largely determined by its formula. That is, the presence of carbon and hydrogen atoms connected to each other.
Mixing acetylene with water, with the addition of catalysts such as mercury salts, leads to the production of acetaldehyde. The triple bond of the atoms contained in the acetylene molecule leads to the fact that during combustion it releases 14,000 kcal/cu. m. During the combustion process, the temperature rises to 3000 °C.
This gas, under certain conditions, can turn into benzene. To do this, you need to heat it to 4000 °C and add graphite.
The hydrogen contained in the molecules shows acidic properties. That is, they are quite easily separated from the molecule in the form of protons. Acetylene is able to decolorize water containing bromine and a solution of potassium permanganate.
The molar mass of acetylene is 26.04 g/mol. Acetylene density is 1.1 kg/m³.
Physical properties
Under standard conditions, acetylene is a colorless gas that is practically insoluble in water. It begins to boil at -830 °C. When compressed, it begins to decompose, releasing a large amount of energy. Therefore, steel cylinders capable of storing gas under high pressure are used to store it.
This gas must not be released into the atmosphere. Its formula may have a negative impact on the environment.
Chemical properties of acetylene:
The chemical properties of acetylene are similar to those of other representatives of the alkyne series. Therefore, it is characterized by the following chemical reactions:
- 1. halogenation of acetylene:
СH≡CH + Br2 → CHBr=CHBr (1,2-dibromoethene);
CHBr=CHBr + Br2 → CHBr2-CHBr2 (1,1,2,2-tetrabromoethane).
The reaction proceeds stepwise with the formation of alkane derivatives.
During this reaction, acetylene decolorizes bromine water.
- 2. hydrohalogenation of acetylene:
СH≡CH + HBr → CH2=CHBr (bromoethene).
- 3. hydration of acetylene (reaction of Mikhail Grigorievich Kucherov, 1881):
CH≡CH + H2O → [CH2=CH-OH] (enol) → CH3-CH=O (acetic aldehyde) (kat = HgSO4, Hg(NO3)2).
- 4. trimerization of acetylene (reaction of Nikolai Dmitrievich Zelinsky, 1927):
3CH≡CH → C6H6(benzene) (kat = activated carbon, to = 450-500 oC).
The acetylene trimerization reaction is a special case of the acetylene polymerization and occurs when acetylene is passed over activated carbon at a temperature of 450-500 oC.
- 5. acetylene dimerization:
СH≡CH + СH≡CH → CH2=CH-С≡CH (vinylacetylene) (kat = aqueous solution of CuCl and NH4Cl).
The acetylene dimerization reaction is a special case of the acetylene polymerization reaction.
- 6. acetylene combustion:
2СH≡CH + 5О2 → 4СО2 + 2H2О.
Acetylene burns with a white, bright flame.
- 7. oxidation of acetylene.
The course of a reaction and its products are determined by the environment in which it occurs.
- 8. acetylene reduction:
CH≡CH + H2 → C2H4 (ethylene) (kat = Ni, Pd or Pt, increased to);
CH≡CH + 2H2 → C2H6 (ethane) (kat = Ni, Pd or Pt, increased to).
Welding technology and modes
Acetylene-oxygen mixtures are used to join parts made of carbon and low-alloy steels. For example, this method is widely used to create permanent pipeline connections. For example, pipes with a diameter of 159 mm with a wall thickness of no more than 8 mm. But there are also some restrictions; joining steel grades 12×2M1, 12×2MFSR using this method is unacceptable.
Selecting mode parameters
To prepare the mixture necessary for combining metals, use the formula 1/1,2. When processing workpieces made of alloy steels, the welder must monitor the state of the flame. In particular, an excess of acetylene should not be allowed.
The consumption of the mixture with the oxygen/acetylene formula is 100-130 dm 3 /hour per 1 mm of thickness. The flame power is regulated using a burner, which is selected depending on the material used, its characteristics, thickness, etc.
To perform welding with acetylene, welding wire is used. Its grade must correspond to the steel grade of the parts being welded. The diameter of the wire is determined depending on the thickness of the metal being welded.
For the convenience of technologists and welders themselves, there are many tables on the basis of which you can quite easily select a welding mode. To do this you need to know the following parameters:
- wall thickness of welded workpieces;
- type of welding - left, right;
Read also: The tire on the chainsaw gets hot
Based on this, you can determine the diameter of the filler wire and select the acetylene consumption. For example, the thickness is 5-6 mm, tip No. 4 will be used to perform the work. That is, based on the tabular data, the wire diameter will be 3.5 mm for left welding, 3.5 mm for right welding. Acetylene consumption in this case will be for left welding method 60-780 dm 3 / hour, with the right 650-750 dm 3 / hour.
Welding is performed in small sections of 10-15 mm. The work is performed in the following sequence. At the first stage, the edges are melted. After this, the root suture is applied. Once the root formation is complete, welding can continue. If the thickness of the workpieces is 4 mm, then welding can be performed in one layer. If the thickness exceeds the specified one, then a second one must be applied. It is laid only after the root of the seam has been completed along the entire specified length.
To improve the quality of welding, preheating is allowed. That is, the future welded joint is heated using a torch. If this method is adopted as a basis, then warming up must be done again after each stop.
Seams can be made with gas in any spatial position. For example, when making a vertical seam there are some peculiarities. So, the vertical seam should be made from bottom to top.
When performing welding work, breaks in work are unacceptable, at least until the entire seam is cut. When stopping operation, the burner must be withdrawn slowly, otherwise seam defects - cavities and pores - may occur. An interesting feature exists when welding pipelines; a draft is not allowed in it and therefore the ends of the pipes must be plugged.
Aldehyde oxidation
The oxidation of aldehydes also produces carboxylic acids or their salts. Aldehydes react with a solution of potassium permanganate or dichromate in an acidic medium when heated, as well as with copper hydroxide when heated.
| For example, when acetaldehyde is oxidized with potassium permanganate in sulfuric acid, acetic acid is formed. |
| For example, the oxidation of aldehydes with copper (II) hydroxide also produces carboxylic acids |
Esters are obtained in the laboratory by reacting carboxylic acids with monohydric and polyhydric alcohols (esterification reaction).
| For example, ethanol reacts with acetic acid to form ethyl acetate (ethyl acetate): |
Types of acetylene
The industry produces two types of acetylene - solid and gas.
Gaseous
Acetylene has a pungent odor and this provides certain advantages in case of leakage. Its mass is close to that of atmospheric air.
Liquid
Liquid acetylene has no color. It has one feature: it refracts color. Acetylene, both liquid and gaseous, is a dangerous substance. That is, if the rules for handling it are violated, an explosion can occur at any second, even at room temperature. To increase safety when handling it, so-called phlegmatization is used. That is, a porous substance is placed in a container intended for acetylene storage. Which reduces its danger
Acetylene molecule models
Formulas showing the distribution of electrons served as the foundation for the creation of atomic orbital models and spatial formulas of molecules (stereochemical). At the end of the 18th century, ball-and-stick models became widespread - for example, balls of different colors and sizes, indicating carbon and hydrogen, which form acetylene. The structural formula of a molecule is presented in the form of rods, symbolizing chemical bonds and their number in each atom.
The ball-and-stick model of acetylene reproduces bond angles equal to 180°, but the internuclear distances in the molecule are reflected approximately. The voids between the balls do not create the idea of filling the space of atoms with electron density. The shortcoming is eliminated in Dreiding's models, which designate the nuclei of atoms not as balls, but as points of attachment of rods to each other. Modern three-dimensional models provide a clearer picture of atomic and molecular orbitals.