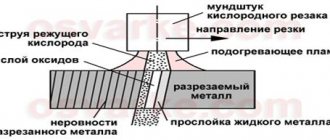
Metals are the most common material (along with plastics and glass) that has been used by people since ancient times. Even then, man knew the characteristics of metals; he profitably used all their properties to create beautiful works of art, dishes, household items, and structures.
One of the main features when considering these substances is their hardness and refractoriness. It is these qualities that make it possible to determine the area of use of a particular metal. Therefore, we will consider all the physical properties and pay special attention to the issues of fusibility.
Physical properties of metals
The characteristics of metals by physical properties can be expressed in the form of four main points.
- Metallic luster - all have approximately the same silvery-white beautiful characteristic luster, except for copper and gold. They have a reddish and yellow tint, respectively. Calcium is silvery blue.
- Physical state - all solids under ordinary conditions, except mercury, which is in the form of a liquid.
- Electrical and thermal conductivity is characteristic of all metals, but is expressed to varying degrees.
- Malleability and ductility are also parameters common to all metals, which can vary depending on the specific representative.
- Melting and boiling points determine which metal is refractory and which is fusible. This parameter is different for all elements.
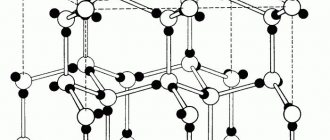
All physical properties are explained by the special structure of the metal crystal lattice. Its spatial arrangement, shape and strength.
What is melting point
Each metal has unique properties, and this list includes its melting point.
When melting, the metal changes from one state to another, namely, from solid to liquid. To fuse a metal, you need to bring heat closer to it and heat it to the required temperature - this process is called the melting point. At the moment when the temperature reaches the desired level, it can still remain in a solid state. If you continue the impact, the metal or alloy will begin to melt. Melting and boiling are not the same thing. The point at which a substance transitions from solid to liquid is often referred to as the melting point of the metal. In the molten state, the molecules do not have a specific arrangement, but attraction holds them close together; in liquid form, the crystalline body leaves volume, but the shape is lost.
During boiling, the volume is lost, the molecules interact very weakly with each other, move chaotically in different directions, and separate from the surface. Boiling point is the process by which the pressure of metal vapor is equal to the pressure of the external environment.
In order to simplify the difference between critical heating points, we have prepared a simple table for you:
| Property | Melting temperature | Boiling temperature |
| Physical state | The alloy transforms into a melt, the crystalline structure is destroyed, and granularity passes through | Transforms into a gas state, some molecules can fly away outside the melt |
| Phase transition | Equilibrium between solid and liquid | Pressure equilibrium between metal vapor and air |
| Influence of external pressure | No changes | There are changes, the temperature decreases with discharge |
Low-melting and refractory metals
This parameter is very important when it comes to the areas of application of the substances in question. Refractory metals and alloys are the basis of machine and shipbuilding, smelting and casting of many important products, and obtaining high-quality working tools. Therefore, knowledge of melting and boiling points plays a fundamental role.
Characterizing metals by strength, we can divide them into hard and brittle. If we talk about refractoriness, then there are two main groups:
- Low-fusibility ones are those that are capable of changing their state of aggregation at temperatures below 1000 °C. Examples include: tin, lead, mercury, sodium, cesium, manganese, zinc, aluminum and others.
- Refractory are those whose melting point is higher than the indicated value. There are not many of them, and even fewer are used in practice.
A table of metals with a melting point above 1000 °C is presented below. This is where the most refractory representatives are located.
| Metal name | Melting point, oC | Boiling point, oC |
| Gold, Au | 1064.18 | 2856 |
| Beryllium, Be | 1287 | 2471 |
| Cobalt, Co | 1495 | 2927 |
| Chromium, Cr | 1907 | 2671 |
| Copper, Cu | 1084,62 | 2562 |
| Iron, Fe | 1538 | 2861 |
| Hafnium, Hf | 2233 | 4603 |
| Iridium, Ir | 2446 | 4428 |
| Manganese, Mn | 1246 | 2061 |
| Molybdenum, Mo | 2623 | 4639 |
| Niobium, Nb | 2477 | 4744 |
| Nickel, Ni | 1455 | 2913 |
| Palladium, Pd | 1554,9 | 2963 |
| Platinum, Pt | 1768.4 | 3825 |
| Rhenium, Re | 3186 | 5596 |
| Rhodium, Rh | 1964 | 3695 |
| Ruthenium, Ru | 2334 | 4150 |
| Tantalus, Ta | 3017 | 5458 |
| Technetium, Ts | 2157 | 4265 |
| Thorium, Th | 1750 | 4788 |
| Titanium, Ti | 1668 | 3287 |
| Vanadium, V | 1910 | 3407 |
| Tungsten, W | 3422 | 5555 |
| Zirconium, Zr | 1855 | 4409 |
This table of metals includes all representatives whose melting point is above 1000 °C. However, in practice, many of them are not used for various reasons. For example, due to economic benefits or due to radioactivity, too high a degree of fragility, susceptibility to corrosive effects.
It is also obvious from the table data that the most refractory metal in the world is tungsten. Gold has the lowest rate. When working with metals, softness is important. Therefore, many of the above are also not used for technical purposes.
What is the refractoriness of metals?
The essence of the term should be clear from the phrase itself - these are metals that “tightly”/hard to melt. In most scientific and technical literature, the term is assigned based on the minimum melting point of a chemical element - from +2,200 degrees Celsius.
Additional chemical elements belong to the so-called extended group of refractory metals - 9 substances + 5 from the main group. There are other metals whose melting point falls within the range of refractory substances, but they are located in the periodic table behind uranium (transuranium).
Due to the instability of isotopes + small distribution over the earth's surface, transuranium metals are not classified as refractory metals.
Physical/chemical properties of refractory metals:
- if we do not take into account carbon and osmium, the temperature indicators for melting of substances have no competitors in the periodic table;
- a high threshold of resistance to deformation of a substance under constant influence of mechanical pressure (creep deformation). For ordinary metal elements, the specified threshold starts from 220 degrees, and for refractory ones from 15,000 degrees. That is why it is much easier to forge iron than a product made from niobium or other refractory substance;
- due to the ease of reaction of compounds with other chemical elements, it is almost impossible to find refractory metals in their pure form;
- In the open air, refractory metals oxidize very slowly. Almost immediately a protective layer in the form of a film forms on the surface;
- When infusible metals are heated, they become vulnerable to corrosion. Their fragility increases + 50%+ of their properties are lost.
The physical properties of refractory metals vary greatly due to their belonging to different groups. All 100% of the elements are refractory, but only 25% of them can be classified as heat-resistant. This difference is due to a change in physical properties when the chemical element is heated. The metal may become susceptible to aggressive environments such as alkalis and acids. More details on each of the refractory metals will be below.
The most refractory metal is tungsten
In the periodic table it is located at serial number 74. It was named after the famous physicist Stephen Wolfram. Under normal conditions, it is a hard, refractory metal with a silvery-white color. Has a pronounced metallic luster. Chemically practically inert, it reacts reluctantly.
Found in nature in the form of minerals:
- wolframite;
- scheelitis;
- hübnerite;
- ferberite
Scientists have proven that tungsten is the most refractory metal of all existing ones. However, there are suggestions that seaborgium is theoretically capable of breaking the record of this metal. But it is a radioactive element with a very short lifetime. Therefore, it is not yet possible to prove this.
At a certain temperature (over 1500 °C), tungsten becomes malleable and ductile. Therefore, it is possible to produce thin wire based on it. This property is used to make filaments in ordinary household light bulbs.
As the most refractory metal, withstanding temperatures above 3400 °C, tungsten is used in the following areas of technology:
- as an electrode for argon welding;
- for producing acid-resistant, wear-resistant and heat-resistant alloys;
- as a heating element;
- in vacuum tubes as filament and so on.
In addition to metal tungsten, its compounds are widely used in technology, science and electronics. As the most refractory metal in the world, it forms compounds with very high-quality characteristics: strong, resistant to almost all types of chemical influences, non-corrosive, and can withstand low and high temperatures (tungsten sulfide, its single crystals and other substances will win).
Characteristics of the densest metal
Scientists agreed that, despite almost the same density, iridium is only slightly inferior to the heaviest metal. However, the physicochemical properties of these two elements have not yet been fully studied.
The rarity and labor-intensive nature of extraction determine the cost of osmium - on average, $15,000 per gram. It is included in the platinum group and is conventionally considered noble, but the name of the metal contradicts its status: in Greek “osme” means “smell”. Due to its high chemical activity, osmium smells like a mixture of garlic or radish with chlorine.
Solidifying from the melt, osmium forms beautiful crystals with an interesting blue or silver-blue tint. But, despite its beauty, it is not suitable for making precious accessories, since it does not have the properties necessary for jewelers: malleability and plasticity.
The element is valuable only because of its special strength. Alloys to which very small doses of the heaviest metal are added become incredibly wear-resistant. Usually it is used to cover units that are subject to constant friction.
History of discovery
The years 1803-1804 became a turning point for the heaviest metal: it was at this time that its discovery took place practically under competition conditions.
First, the English chemist Smithson Tennant and his assistant William Hyde Wollaston, who made more than one important discovery, discovered an unusual sediment with a characteristic odor during an experiment with platinum ores and nitric and hydrochloric acids and shared their discovery with others. Then the French scientists Antoine de Fourcroy and Louis-Nicolas Vauquelin took over the baton and, based on previous and their own research, announced the discovery of a new element. The name was given to it “pten”, which means “flying”, since as a result of the experiments they received flying black smoke
The name was given to it “pten”, which means “flying”, since as a result of the experiments they received flying black smoke
Then the French scientists Antoine de Fourcroy and Louis-Nicolas Vauquelin took over the baton and, based on previous and their own research, announced the discovery of a new element. The name was given to it “pten”, which means “flying”, since as a result of the experiments they received flying black smoke.
However, Tennant did not sleep either: he continued his research and did not lose sight of the experiments of the French. As a result, Smithson achieved more concrete results and, in an official document sent to the Royal Society of London, indicated that he had divided pten into two related elements: iridium (“rainbow”) and osmium (“smell”).
Where is it used?
The list of areas of application is quite extensive: aviation, military and missile technology, aerospace industry, medicine. Although weapons manufacturers are already thinking about what can replace the heaviest metal in the world, since osmium is too difficult to process.
Almost half of the world's reserves of the heaviest metal are devoted to the needs of the chemical industry. It is used to stain living tissues under a microscope, ensuring their preservation. In addition, it is used as a dye when painting porcelain.
Isotopes of the heaviest metal are used to make containers for storing nuclear waste.
Places of natural occurrence
It is almost impossible to detect osmium in its pure form. This heavy element is usually found in combination with iridium. The substance is contained in deposits of platinum ores and at the crash site or in the meteorites themselves that hit the Earth.
Niobium and its alloys
Nb, or niobium, is a silvery-white shiny metal under normal conditions. It is also refractory, since the temperature of transition to the liquid state for it is 2477 °C. It is this quality, as well as the combination of low chemical activity and superconductivity, that allows niobium to become increasingly popular in human practice every year. Today this metal is used in industries such as:
- rocket science;
- aviation and space industry;
- nuclear power;
- chemical apparatus engineering;
- radio engineering.
This metal retains its physical properties even at very low temperatures. Products based on it are characterized by corrosion resistance, heat resistance, strength, and excellent conductivity.
This metal is added to aluminum materials to improve chemical resistance. Cathodes and anodes are made from it, and non-ferrous alloys are alloyed with it. Even coins in some countries are made with niobium content.
What does melting temperature depend on?
For different substances, the temperature at which the structure is completely reconstructed to the liquid state is different. If we take into account metals and alloys, then it is worth noting the following points:
- Metals are not often found in their pure form. The temperature directly depends on its composition. As an example, we will indicate tin, to which other substances (for example, silver) can be added. Impurities make the material more or less resistant to heat.
- There are alloys that, due to their chemical composition, can transform into a liquid state at temperatures above one hundred and fifty degrees. There are also alloys that can “hold” when heated to three thousand degrees and above. Taking into account the fact that when the crystal lattice changes, the physical and mechanical qualities change, and operating conditions can be determined by the heating temperature. It is worth noting that the melting point of a metal is an important property of a substance. An example of this is aviation equipment.
Heat treatment, in most cases, almost does not change the resistance to heat. The only sure way to increase resistance to heat is to make changes to the chemical composition; this is why steel is alloyed.
Tantalum
Metal, in its free form and under normal conditions, covered with an oxide film. It has a set of physical properties that allow it to be widespread and very important for humans. Its main characteristics are as follows:
- At temperatures above 1000 oC it becomes a superconductor.
- It is the most refractory metal after tungsten and rhenium. The melting point is 3017 °C.
- Absorbs gases perfectly.
- It is easy to work with as it can be rolled into sheets, foil and wire without much difficulty.
- It has good hardness and is not brittle, retains ductility.
- Very resistant to chemical agents (does not dissolve even in aqua regia).
Thanks to these characteristics, it has managed to gain popularity as the basis for many heat-resistant and acid-resistant, anti-corrosion alloys. Its numerous compounds are used in nuclear physics, electronics, and computational devices. Used as superconductors. Previously, tantalum was used as an element in incandescent lamps. Now tungsten has taken its place.
Receiving technology
The source of most refractory materials is ore.
The process is traditional:
- Impurities are removed from it.
- Refined (restores the desired element). The recovery method depends on the required degree of metal purity. Therefore, arc-shaped, electron beam or plasma melting is used.
- Plasma produces the best product. It comes in the form of small granules, powder or blanks (wire, foil, ingots, fittings, rolled products).
The melting technology is specific, so special enterprises deal with such raw materials. There were only two of them in the USSR.
Processing of refractory metals is possible only by powder metallurgy methods.
Chrome and its alloys
One of the hardest metals, naturally bluish-white in color. Its melting point is lower than that of the elements considered so far, and is 1907 °C. However, it is still used everywhere in technology and industry, as it lends itself well to mechanical stress, is processed and molded.
Chromium is especially valuable as a coating. It is applied to products to give them a beautiful shine, protect against corrosion and increase wear resistance. The process is called chrome plating.
Chromium alloys are very popular. After all, even a small amount of this metal in the alloy significantly increases the hardness and resistance of the latter to impacts.
Application and presence in nature
The most fusible metal in the world is found very scattered in nature. Its total concentration in the earth's crust is approximately 83 mg/t, which makes it a rather rare element. It is found in large quantities in shales and sulfide minerals, especially sphalerites and stibnites. Found in livingstonites and metacinnabarites.
Despite its toxicity, mercury is used in many fields, for example, in metallurgy, medicine, the chemical industry, mechanical engineering, electrical engineering and even agriculture. The most fusible metal is suitable for filling energy-saving lamps, thermometers and barometers.
In heavy industry, the substance is used for mercury steam turbines, vacuum units and diffusion pumps. It is used to fill measuring instruments, batteries, and dry batteries. Mercury is involved in the production of air conditioners, refrigerators and washing machines. In agriculture, it is used as part of pesticides.
Zirconium
It is one of the most expensive metals, so its use for technical purposes is difficult. However, its physical characteristics make it simply indispensable in many other industries.
Under normal conditions it is a beautiful silvery-white metal. It has a fairly high melting point - 1855 oC. It has good hardness and corrosion resistance, as it is not chemically active. It also has excellent biological compatibility with human skin and the entire body as a whole. This makes it a valuable metal for medical use (instruments, prosthetics, etc.).
The main areas of application of zirconium and its compounds, including alloys, are as follows:
- nuclear energy;
- pyrotechnics;
- metal alloying;
- medicine;
- production of bioware;
- construction material;
- like a superconductor.
Even jewelry that can influence the improvement of human health is made from zirconium and alloys based on it.
Types and applications
Due to their unique properties, refractory metals are very useful for various applications and industries. Their main advantages:
- Ultra-high melting point. In particular, refractory metals include tungsten, molybdenum and tantalum, which are used in the production of glass;
- Strength at ultra-high temperatures. For example, rocket cones made of tungsten have twice the tensile strength of iron at normal temperatures;
- Excellent abrasion and wear resistance to extend the life of valve seats, seals, injectors and other high wear areas;
- Excellent corrosion resistance, which is why critical pipelines in chemical plants are usually made of refractory metals;
- Resistance to thermal shock. In particular, tungsten products can withstand stress caused by rapid expansion due to sudden temperature changes;
- Thermal and electrical conductivity, as a result of which radiator parts are made from tungsten and molybdenum;
- Extreme hardness, therefore highly resistant cutting stamping and drilling tools are made from tungsten carbide;
- The high density of refractory metals is the reason for their use in golf club heads and aircraft gyroscopes.
In addition, these materials are used as catalysts for chemical reactions, in nuclear fusion processes, etc.
Refractory metals include the particularly widespread tungsten, molybdenum, niobium, tantalum, rhenium and chromium. The specifics of their use are discussed below.
Tungsten
Tungsten is the most common refractory metal. It has the highest melting point and one of the highest densities. It is also highly resistant to corrosion. Widely used in wire fibers, such as most incandescent lamps used in homes, as well as industrial arc lamps and other lighting equipment.
Molybdenum
Molybdenum is the most used refractory metal of all because it is cheaper than most others and, when made into an alloy, can be very resistant to creep and high temperatures. It also does not form amalgams, making it resistant to corrosion.
Molybdenum is used to strengthen steel alloys, especially in structural piping and tubing. This metal also has excellent anti-friction properties, making it an ideal component in oils and lubricants used in automobiles.
Niobium
It has an optimal combination of ductility and strength. It can be used in the manufacture of electrolytic capacitors, superconductors, nuclear reactors and vacuum tubes.
Tantalum
It is more resistant to corrosion than others, therefore it is used in medicine (especially surgery), as well as in environments with high acidity. Tantalum is also a major component of computer, telephone and capacitor circuits.
Rhenium
Known for its high tensile strength and ductility. It is widely used in nuclear reactors, gyroscopes and other electrical components. Due to its rarity, rhenium is very expensive. The concept of corrosion resistance is especially relevant for rhenium because it is very volatile. May lose resistance to oxygen at high temperatures as the oxide layer actively evaporates.
Molybdenum
If you find out which metal is the most refractory, then, in addition to the indicated tungsten, you can also name molybdenum. Its melting point is 2623 °C. At the same time, it is quite hard, plastic and amenable to processing.
It is mainly used not in its pure form, but as an integral component of alloys. They, thanks to the presence of molybdenum, are significantly strengthened in wear resistance, heat resistance and anti-corrosion.
Some molybdenum compounds are used as technical lubricants. This metal is also an alloying material that simultaneously affects both strength and corrosion resistance, which is very rare.
Metal melting process
This process refers to the transition of a substance from a solid to a liquid state.
When the melting point is reached, the metal can be in either a solid or liquid state; further increase will lead to the complete transition of the material into a liquid. The same thing happens during solidification - when the melting point is reached, the substance will begin to transition from a liquid to a solid state, and the temperature will not change until complete crystallization.
It should be remembered that this rule applies only to pure metal. Alloys do not have a clear temperature boundary and undergo state transitions in a certain range:
- Solidus is the temperature line at which the most fusible component of the alloy begins to melt.
- Liquidus is the final melting point of all components, below which the first alloy crystals begin to appear.
It is impossible to accurately measure the melting point of such substances; the point of transition of states is indicated by a numerical interval.
Depending on the temperature at which metals begin to melt, they are usually divided into:
- Low-melting, up to 600 °C. These include tin, zinc, lead and others.
- Medium melting, up to 1600 °C. Most common alloys, and metals such as gold, silver, copper, iron, aluminum.
- Refractory, over 1600 °C. Titanium, molybdenum, tungsten, chromium.
There is also a boiling point - the point at which the molten metal begins to transition into a gaseous state. This is a very high temperature, typically 2 times the melting point.
Effect of pressure
The melting temperature and the equal solidification temperature depend on pressure, increasing with its increase.
This is due to the fact that with increasing pressure the atoms come closer to each other, and in order to destroy the crystal lattice they need to be moved away. At increased pressure, greater thermal energy is required and the corresponding melting temperature increases. There are exceptions when the temperature required to transform into a liquid state decreases with increased pressure. Such substances include ice, bismuth, germanium and antimony.
Metal crystal lattices
In its ideal form, it is generally accepted that metals have a cubic lattice (real substances may have flaws). There are equal distances between molecules horizontally and vertically.
A solid substance is characterized by constancy:
- shapes, the object retains linear dimensions in different conditions;
- volume, the object does not change the amount of substance it occupies;
- mass, the amount of a substance expressed in grams (kilograms, tons);
- density, unit volume contains constant mass.
When transitioning into a liquid state, having reached a certain temperature, the crystal lattices are destroyed. Now we can’t talk about constancy of form. The liquid will take the form in which it is poured.
When evaporation occurs, only the mass of the substance remains constant. Gas will take up the entire volume that will be provided to it. Here we cannot say that density is a constant value.
When liquids combine, the following options are possible:
- Liquids completely dissolve in one another, as do water and alcohol. The concentration of substances will be the same throughout the entire volume.
- Liquids are stratified by density, the connection occurs only at the interface. It is only temporarily possible to obtain a mechanical mixture. Mix liquids with different properties. An example is oil and water.
Metals form alloys in the liquid state. To obtain an alloy, each of the components must be in a liquid state. With alloys, phenomena of complete dissolution of one in another are possible. Options cannot be excluded when the alloy will be obtained only as a result of intensive mixing. In this case, the quality of the alloy is not guaranteed, so they try not to mix components that do not allow obtaining stable alloys.
The resulting substances, soluble in each other, when solidified, form crystal lattices of a new type. Define:
- Heliocentered crystal lattices are also called body-centered. In the middle there is a molecule of one substance, and four more molecules of another are located around it. It is customary to call such lattices loose, since the bonds between metal molecules in them are weaker.
- Face-centered crystal lattices form compounds in which the component molecules are located on the faces. Metallurgists call such crystalline alloys dense. In reality, the density of the alloy can be higher than that of each of the components included in the composition (alchemists of the Middle Ages were looking for options for alloys in which the density would correspond to the density of gold).
Strength of metals
In addition to the ability to transition from a solid to a liquid state, one of the important properties of a material is its strength - the ability of a solid body to resist destruction and irreversible changes in shape. The main indicator of strength is the resistance that occurs when a pre-annealed workpiece breaks. The concept of strength does not apply to mercury because it is in a liquid state. The designation of strength is accepted in MPa - Mega Pascals.
There are the following strength groups of metals:
- Fragile. Their resistance does not exceed 50MPa. These include tin, lead, soft-alkaline metals
- Durable, 50−500 MPa. Copper, aluminum, iron, titanium. Materials of this group are the basis of many structural alloys.
- High strength, over 500 MPa. For example, molybdenum and tungsten.
Vanadium
Gray metal with a silvery sheen. It has a fairly high fusibility index (1920 °C). It is used mainly as a catalyst in many processes due to its inertness. It is used in the energy sector as a chemical current source, in the production of inorganic acids. It is not the pure metal that is of primary importance, but rather some of its compounds.
At what temperature does it melt?
Metal elements, whatever they are, melt almost one to one. This process occurs when heated. It can be both external and internal. The first takes place in a furnace, and for the second, resistive heating is used, passing electricity or induction heating. The impact is almost the same. When heated, the amplitude of molecular vibrations increases. Structural lattice defects are formed, which are accompanied by the breaking of interatomic bonds. The process of lattice destruction and the accumulation of such defects means melting.
Different substances have different melting points. Theoretically, metals are divided into:
- Low-melting - temperatures up to 600 degrees Celsius are sufficient to obtain a liquid substance.
- Medium melting - requires a temperature of 600 to 1600 ⁰C.
- Refractory are metals that require temperatures above 1600 ⁰C to melt.
Melting iron
The melting point of iron is quite high. A technically pure element requires a temperature of +1539 °C. This substance contains an impurity - sulfur, and it can only be extracted in liquid form.
Without impurities, pure material can be obtained by electrolysis of metal salts.
Melting cast iron
Cast iron is the best metal for smelting. The high fluidity rate and low shrinkage rate make it possible to use it more effectively during casting. Next, consider the boiling point of cast iron in degrees Celsius:
- Gray - the temperature can reach 1260 degrees. When pouring into molds, the temperature can rise to 1400.
- White - the temperature reaches 1350 degrees. It is poured into molds at 1450.
Melting steel
Steel is an alloy of iron mixed with carbon. Its main benefit is strength, since this substance is capable of maintaining its volume and shape for a long time. This is due to the fact that the particles are in an equilibrium position. Thus, the forces of attraction and repulsion between particles are equal.
Melting aluminum and copper
The melting point of aluminum is 660 degrees, which means that it can be melted at home.
Pure copper is 1083 degrees, and for copper alloys it ranges from 930 to 1140 degrees.
Rhenium and alloys based on it
Which metal is the most refractory after tungsten? This is rhenium. Its fusibility index is 3186 °C. It is superior in strength to both tungsten and molybdenum. Its plasticity is not too high. The demand for rhenium is very high, but production is difficult. As a result, it is the most expensive metal existing today.
Used for making:
- jet engines;
- thermocouples;
- filaments for spectrometers and other devices;
- as a catalyst in oil refining.
All areas of application are expensive, so it is used only in cases of extreme necessity, when there is no possibility of replacing it with anything else.
Melting point of non-metals
Non-metallic materials can be presented in solid and liquid form. Inorganic substances are presented in table. 4.
Table 4, melting point of inorganic non-metals:
In practice, organic materials are of greatest interest to users: polyethylene, polypropylene, wax, paraffin and others. The melting points of some substances are shown in table. 5.
Table 5, melting temperature of polymer materials:
Attention! The glass transition temperature refers to the state at which a material becomes brittle.
Video: melting point of known metals.
Sources
- https://dosaafvlg-kotovo.ru/stanki-drugoe/temperatura-kipeniya-stali.html
- https://tutsvarka.ru/vidy/temperatura-plavleniya-metallov-tablitsa-i-ponyatie
- https://zpu-tmb.ru/metalloprokat/pri-kakoj-temperature-plavitsya-metall.html
- https://ru.wikipedia.org/wiki/%D0%A2%D0%B5%D0%BC%D0%BF%D0%B5%D1%80%D0%B0%D1%82%D1%83%D1 %80%D0%B0_%D0%BF%D0%BB%D0%B0%D0%B2%D0%BB%D0%B5%D0%BD%D0%B8%D1%8F
- https://pressadv.ru/stali/temperatura-plavleniya-metallov-tablica.html
- https://tpspribor.ru/vidy-metalla/pri-kakoy-temperature-plavitsya-metall-v-gradusah.html
- https://metmastanki.ru/temperatura-plavleniya-metallov-i-nemetallov-tablitsy
- https://svarkaprosto.ru/tehnologii/pri-kakoj-temperature-plavitsya-metall
- https://morflot.su/temperatura-plavlenija-metallov-tablica-po/
- https://stanok.guru/stanki/metallorezhuschiy-stanok/temperatura-plavleniya-raznyh-metallov-v-tablice.html
- https://plazmen.ru/kakova-temperatura-plavleniya-zheleza/
How is tungsten obtained?
Pure tungsten does not occur in nature. It is part of rocks in the form of trioxide, as well as wolframites of iron, manganese and calcium, less often copper or lead. According to scientists, the tungsten content in the earth's crust averages 1.3 grams per ton. This is a rather rare element compared to other types of metals. The tungsten content in ore after mining usually does not exceed 2%. Therefore, the extracted raw materials are sent to processing plants, where the mass fraction of metal is brought to 55-60% using magnetic or electrostatic separation.
The process of its production is divided into technological stages. In the first stage, pure trioxide is isolated from the mined ore. For this purpose, the thermal decomposition method is used. At temperatures from 500 to 800 degrees Celsius, all excess elements melt, and refractory tungsten in the form of oxide can be easily collected from the melt. The output is raw material with a hexavalent tungsten oxide content of 99%.
The resulting compound is thoroughly crushed and a reduction reaction is carried out in the presence of hydrogen at a temperature of 700 degrees Celsius. This allows you to isolate pure metal in powder form. Next, it is pressed under high pressure and sintered in a hydrogen environment at temperatures of 1200-1300 degrees Celsius. After this, the resulting mass is sent to an electric melting furnace, where, under the influence of current, it is heated to a temperature of over 3000 degrees. This is how tungsten turns into a molten state.
For final purification from impurities and obtaining a single-crystal structural lattice, the zone melting method is used. It implies that at a certain point in time only a certain zone of the total area of the metal is molten. Gradually moving, this zone redistributes impurities, as a result of which they ultimately accumulate in one place and can be easily removed from the alloy structure.
Finished tungsten arrives at the warehouse in the form of bars or ingots, intended for the subsequent production of the desired products. To obtain tungsten alloys, all constituent elements are crushed and mixed in powder form in the required proportions. Next, sintering and melting are carried out in an electric furnace.