History of the discovery of oxygen
The discovery of oxygen is credited to Joseph Priestley. He had a laboratory equipped with instruments for collecting gases. He tested its physiological effects on himself and on mice. Priestley found that after inhaling the gas, a pleasant lightness was felt for some time. Mice in a hermetically sealed jar of air suffocate faster than in a jar of O2. Since Priestley was an adherent of the phlogiston theory, he never found out what was in his hands. He only described this gas, without even realizing what he was describing. But the laurels of the discovery of oxygen belong to Antoine Laurent de Lavoisier, who gave it its name.
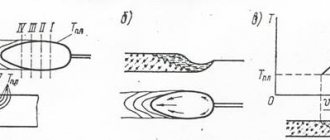
Lavoisier staged his famous experiment, which lasted 12 days. He heated mercury in a retort. When boiling, its red oxide was formed. When the retort was cooled, it turned out that the air in it had decreased by almost 1/6 of its volume, and the remainder of the mercury weighed less than before heating. But when the mercury oxide was decomposed by strong calcination, everything returned: both the lack of mercury and the “disappeared” oxygen.
Subsequently, Lavoisier established that this gas is part of nitric, sulfuric, and phosphoric acids. He mistakenly believed that O2 was necessarily part of acids, and therefore called it “oxygenium,” which means “acid-producing.” Nowadays, acids devoid of “oxygenium” are well known (for example: hydrochloric, hydrogen sulfide, hydrocyanic, etc.).
How much does air weigh?
- home
- Directory
- units of measurement
- Mass and weight
- How much does air weigh?
- Air composition
- Nitrogen
- Oxygen
- Argon
- Carbon dioxide
- Neon
- Methane
- Helium
- Krypton
- Hydrogen
- Xenon
- How much does a cube of air weigh?
- How much does a liter of air weigh?
- Physical properties of air
Air is an intangible quantity, it cannot be touched or smelled, it is everywhere, but for humans it is invisible, it is not easy to find out how much air weighs, but it is possible. If the surface of the Earth, as in a children's game, is drawn into small squares measuring 1x1 cm, then the weight of each of them will be equal to 1 kg, that is, 1 cm2 of atmosphere contains 1 kg of air.
Can this be proven? Quite. If you build a scale from an ordinary pencil and two balloons, securing the structure to a thread, the pencil will be in balance, since the weight of the two inflated balloons is the same. Once one of the balloons is pierced, the advantage will be in the direction of the inflated balloon, because the air from the damaged balloon has escaped. Accordingly, simple physical experience proves that air has a certain weight. But, if you weigh the air on a flat surface and in the mountains, its mass will turn out to be different - mountain air is much lighter than the air we breathe near the sea. There are several reasons for the different weights:
Air composition
The weight of 1 m3 of air is 1.29 kg.
Can it be proven that air has weight? Quite. If you build a scale from an ordinary pencil and two balloons, securing the structure to a thread, the pencil will be in balance, since the weight of the two inflated balloons is the same. Once one of the balloons is pierced, the advantage will be in the direction of the inflated balloon, because the air from the damaged balloon has escaped. Accordingly, simple physical experience proves that air has a certain weight. But, if you weigh the air on a flat surface and in the mountains, its mass will turn out to be different - mountain air is much lighter than the air we breathe near the sea.
There are several reasons for the different weights:
- the higher the air rises, the more rarefied it becomes, that is, high in the mountains, the air pressure will not be 1 kg per cm2, but half as much, but the content of oxygen necessary for breathing also decreases by exactly half, which can cause dizziness and nausea and ear pain;
- water content in the air.
The air mixture includes:
- Nitrogen – 75.5%;
- Oxygen – 23.15%;
- Argon – 1.292%;
- Carbon dioxide – 0.046%;
- Neon – 0.0014%;
- Methane – 0.000084%;
- Helium – 0.000073%;
- Krypton – 0.003%;
- Hydrogen – 0.00008%;
- Xenon – 0.00004%.
Let's consider what are the gases that form air?
Nitrogen
The nitrogen content in the air is 78% by volume and 75% by mass, that is, this element dominates in the atmosphere, has the title of one of the most common on Earth, and, in addition, is found outside the human habitation zone - on Uranus, Neptune and in interstellar spaces. So, we have already figured out how much nitrogen is in the air, but the question remains about its function. Nitrogen is necessary for the existence of living beings, it is part of:
- proteins;
- amino acids;
- nucleic acids;
- chlorophyll;
- hemoglobin, etc.
On average, about 2% of a living cell consists of nitrogen atoms, which explains why there is so much nitrogen in the air as a percentage of volume and mass. Nitrogen is also one of the inert gases extracted from atmospheric air. Ammonia is synthesized from it and used for cooling and other purposes.
Oxygen
The oxygen content in the air is one of the most popular questions. Keeping the intrigue, let's digress to one fun fact: oxygen was discovered twice - in 1771 and 1774, however, due to the difference in publications of the discovery, the honors of discovering the element went to the English chemist Joseph Priestley, who actually isolated oxygen second. So, the proportion of oxygen in the air fluctuates around 21% by volume and 23% by mass. Together with nitrogen, these two gases form 99% of all earth's air. However, the percentage of oxygen in the air is less than nitrogen, and yet we do not experience breathing problems. The fact is that the amount of oxygen in the air is optimally calculated specifically for normal breathing; in its pure form, this gas acts on the body like poison, leading to difficulties in the functioning of the nervous system, disruption of breathing and blood circulation. At the same time, the lack of oxygen also negatively affects health, causing oxygen starvation and all the unpleasant symptoms associated with it. Therefore, how much oxygen is contained in the air is what is needed for healthy, full breathing.
Argon
Argon ranks third in the air; it is odorless, colorless and tasteless. No significant biological role of this gas has been identified, but it has a narcotic effect and is even considered doping. Argon extracted from the atmosphere is used in industry, medicine, to create an artificial atmosphere, chemical synthesis, fire extinguishing, creating lasers, etc.
Carbon dioxide
Carbon dioxide makes up the atmosphere of Venus and Mars; its percentage in the earth's air is much lower. At the same time, a huge amount of carbon dioxide is contained in the ocean, it is regularly supplied by all breathing organisms, and is released due to the work of industry. In human life, carbon dioxide is used in fire fighting, the food industry as a gas and as a food additive E290 - a preservative and leavening agent. In solid form, carbon dioxide is one of the most well-known refrigerants, “dry ice.”
Neon
That same mysterious light of disco lights, bright signs and modern headlights use the fifth most common chemical element, which is also inhaled by humans - neon. Like many inert gases, neon has a narcotic effect on humans at a certain pressure, but it is this gas that is used in the training of divers and other people working at high pressure. Also, neon-helium mixtures are used in medicine for respiratory disorders; neon itself is used for cooling, in the production of signal lights and those same neon lamps. However, contrary to the stereotype, neon light is not blue, but red. All other colors are produced by lamps with other gases.
Methane
Methane and air have a very ancient history: in the primary atmosphere, even before the appearance of man, methane was in much greater quantities. Now extracted and used as fuel and raw material in manufacturing, this gas is not as widespread in the atmosphere, but is still released from the Earth. Modern research establishes the role of methane in the respiration and vital functions of the human body, but there is no authoritative data on this yet.
Helium
Having looked at how much helium is in the air, anyone will understand that this gas is not one of the most important. Indeed, it is difficult to determine the biological significance of this gas. Apart from the funny distortion of the voice when inhaling helium from a balloon. However, helium is widely used in industry: in metallurgy, the food industry, for filling aircraft and weather probes, in lasers, nuclear reactors, etc.
Krypton
This is not about Superman's homeland. Krypton is a noble gas that is three times heavier than air, chemically inert, extracted from air, used in incandescent light bulbs, lasers, and is still being actively studied. Among the interesting properties of krypton, it is worth noting that at a pressure of 3.5 atmospheres it has a narcotic effect on humans, and at 6 atmospheres it acquires a pungent odor.
Hydrogen
Hydrogen in the air occupies 0.00005% by volume and 0.00008% by mass, but at the same time it is the most common element in the Universe. It is quite possible to write a separate article about its history, production and application, so now we will limit ourselves to a small list of industries: chemical, fuel, food industries, aviation, meteorology, electric power.
Xenon
The latter is a component of air, which was initially considered only an admixture of krypton.
Its name translates as “alien”, and the percentage of content both on Earth and beyond is minimal, which led to its high cost. Nowadays they cannot do without xenon: the production of powerful and pulsed light sources, diagnostics and anesthesia in medicine, spacecraft engines, rocket fuel. In addition, when inhaled, xenon significantly lowers the voice (the opposite effect of helium), and recently inhalation of this gas has been included in the list of doping agents. The amount of ingredients in the air may change and, accordingly, the mass of air also undergoes changes in the direction of increase or decrease.
- air always contains water vapor. The physical law is that the higher the air temperature, the more water it contains. This indicator is called air humidity and affects its weight.
What is the weight of air measured in? There are several indicators that determine its mass.
How much does a cube of air weigh?
At a temperature of 0° Celsius, the weight of 1 m3 of air is 1.29 kg.
That is, if you mentally allocate a space in a room with a height, width and length equal to 1 m, then this air cube will contain exactly this amount of air.
If air has weight and weight that is quite noticeable, why does a person not feel heaviness? A physical phenomenon such as atmospheric pressure means that every inhabitant of the planet is pressed by an air column weighing 250 kg. The average palm area of an adult is 77 cm2. That is, in accordance with physical laws, each of us holds 77 kg of air in the palm of our hand! This is equivalent to the fact that we constantly carry 5 pound weights in each hand. In real life, even a weightlifter cannot do this, however, each of us can cope with such a load easily, because atmospheric pressure presses from both sides, both outside the human body and from the inside, that is, the difference is ultimately zero.
The properties of air are such that it affects the human body differently. High in the mountains, due to a lack of oxygen, people experience visual hallucinations, and at great depths, the combination of oxygen and nitrogen in a special mixture - “laughing gas” - can create a feeling of euphoria and a feeling of weightlessness.
Knowing these physical quantities, we can calculate the mass of the Earth’s atmosphere - the amount of air that is held in the near-Earth space by gravitational forces. The upper boundary of the atmosphere ends at an altitude of 118 km, that is, knowing the weight of m3 of air, you can divide the entire surface area into air columns, with a base of 1x1 m, and add up the resulting mass of such columns. Ultimately, it will be equal to 5.3 * 1015 tons. The weight of the planet's air armor is quite large, but it is only one millionth of the total mass of the globe. The Earth's atmosphere serves as a kind of buffer that protects the Earth from unpleasant cosmic surprises. From solar storms alone that reach the surface of the planet, the atmosphere loses up to 100 thousand tons of its mass per year! Such an invisible and reliable shield is air.
How much does a liter of air weigh?
A person does not notice that he is constantly surrounded by transparent and almost invisible air. Is it possible to see this intangible element of the atmosphere? Visually, the movement of air masses is broadcast daily on the television screen - a warm or cold front brings long-awaited warming or heavy snowfall.
What else do we know about air? Probably, the fact that it is vitally necessary for all living beings living on the planet. Every day a person inhales and exhales about 20 kg of air, a quarter of which is consumed by the brain.
The weight of air can be measured in different physical units, including liters.
The weight of one liter of air will be equal to 1.2930 grams, at a pressure of 760 mm Hg. column and a temperature of 0°C.
In addition to the usual gaseous state, air can also be found in liquid form. To transform a substance into this state of aggregation, exposure to enormous pressure and very low temperatures will be required. Astronomers suggest that there are planets whose surfaces are completely covered with liquid air.
The sources of oxygen necessary for human existence are the Amazon forests, which produce up to 20% of this important element on the entire planet.
Forests are truly the “green” lungs of the planet, without which human existence is simply impossible. Therefore, living indoor plants in an apartment are not just a piece of furniture, they purify the indoor air, the pollution of which is tens of times higher than outside.
Clean air has long become a shortage in megacities; air pollution is so great that people are ready to buy clean air. “Air sellers” first appeared in Japan. They produced and sold clean air in cans, and any resident of Tokyo could open a can of clean air for dinner and enjoy its freshest aroma.
Air purity has a significant impact not only on human health, but also on animal health. In polluted areas of equatorial waters, near human-populated areas, dozens of dolphins are dying. The cause of death for mammals is a polluted atmosphere; in autopsies of animals, the lungs of dolphins resemble the lungs of miners, clogged with coal dust. The inhabitants of Antarctica, penguins, are also very sensitive to air pollution; if the air contains a large amount of harmful impurities, they begin to breathe heavily and intermittently.
For a person, clean air is also very important, so after working in the office, doctors recommend taking daily hour-long walks in the park, forest, or outside the city. After such “air” therapy, the body’s vitality is restored and well-being significantly improves. The recipe for this free and effective medicine has been known since ancient times; many scientists and rulers considered daily walks in the fresh air a mandatory ritual.
For a modern city dweller, air treatment is very relevant: a small portion of life-giving air, weighing 1-2 kg, is a panacea for many modern ailments!
Physical properties of air
Like any mixture of substances, today it is possible to determine the physical properties of air.
| Property | Meaning |
| Average molar mass (average mass of one mole of a substance) | 28.98 g/mol |
| Density of dry air at normal atmospheric pressure | 1.29 kg/m3 |
| Average specific heat capacity at constant pressure | 1.005 kJ / (kg K) |
| Average specific heat capacity at constant volume | 0.717 kJ/(kg K) |
| Adiabatic exponent (ratio of heat capacity at constant pressure to heat capacity at constant volume) | 1,40 |
| Thermal conductivity at 0℃ | 0.0243 W/(mK) |
| Speed of sound in air under normal conditions | 331 m/s (1193 km/h) |
| Average coefficient of thermal expansion in the temperature range 0–100°C (volume change with a gradual increase in temperature at constant pressure) | 3.67 10−3 1/K |
| The coefficient of dynamic viscosity of air under normal conditions (dynamic viscosity is the internal resistance of molecules to movement within a substance according to Newton’s law) | 17.2 µPa s |
| Solubility of air in water | 29.18 cm3/l |
| Refractive index under normal conditions (refractive index means the change in the angle of motion of light and any other waves in a substance) | 1,0002926 |
| Refractive index change factor | 2.8 10−9 1/Pa |
| Average polarizability of a molecule | 1,7·10−30 |
Mass and WeightMass Theory Units of Measurement
More interesting things in telegram @calcsbox
Methods for producing oxygen
Oxygen is mainly obtained in three ways:
- air separation by low-temperature rectification (deep cooling);
- decomposition of water by electrolysis (passing electric current);
- chemical method.
It is obtained from atmospheric air by deep cooling, as a by-product in the production of nitrogen.
O2 is also produced by passing an electric current through water (water electrolysis) and producing hydrogen along the way.
The chemical method of production is low-productive and, therefore, uneconomical; it has not found wide application and is used in laboratory practice.
Probably many people remember the chemical experiment when potassium permanganate (potassium permanganate KMnO4) is heated in a flask, and then the gas released during the heating process is collected in another flask?
2KMnO4 = K2MnO4 + MnO2 + O2 ↑
And the whole trick was when a smoldering splinter was placed in this flask and it flared up with a bright flame and the teacher explained that the gas released was O2, which supported combustion. And that the combustion process is nothing more than an oxidation process.
Application of oxygen
In addition to the fact that all living beings in nature, with the exception of a few microorganisms, consume oxygen when breathing, it is widely used in many industries: metallurgical, chemical, mechanical engineering, aviation, rocket science and even in medicine.
In the chemical industry it is used:
- when producing acetylene from natural gas (methane);
- in the production of acids (nitric, sulfuric);
- for gasification of solid fuel;
- for the production of ammonia, formaldehyde and methanol.
In metallurgy it is used:
- when obtaining non-ferrous metals from ores;
- when smelting cast iron in blast furnaces;
- when smelting steel in open-hearth and electric furnaces;
- oxygen-converter steel smelting.
For medical purposes, patients whose normal functioning of the respiratory or circulatory system is disrupted are artificially increased the O2 content in the air or allowed to breathe pure O2 for a short time. Medical oxygen produced by GOST 5583 is especially carefully purified from all impurities.
Use of oxygen in welding
O2 itself is a non-flammable gas, but due to its ability to actively support combustion and increase the intensity (intensification) of combustion of gases and liquid fuels, it is used in rocket power plants and in all gas-flame processing processes. In gas-flame processing processes such as gas welding and surface hardening, a high flame temperature is achieved by burning flammable gases in O2, and in gas cutting, it causes oxidation and combustion of the metal being cut.
In semi-automatic welding (MIG/MAG), oxygen O2 is used as a component of protective gas mixtures with argon (Ar) or carbon dioxide (CO2).
Oxygen is added to argon during semi-automatic welding of alloy steels to ensure stability of the arc and jet transfer of molten metal into the weld pool. The fact is that, as a surface active element, it reduces the surface tension of the liquid metal, promoting the formation of smaller droplets at the end of the electrode.
When semi-automatically welding low-alloy and low-carbon steels, O2 is added to carbon dioxide to ensure deep penetration and good formation of the weld, as well as to reduce spatter.
Most often, oxygen is used in gaseous form, and in liquid form it is used only during its storage and transportation from the manufacturer to consumers.
Gas cylinders
TD Techno-Nova LLC offers gas welding cylinders from a warehouse in Moscow for use in everyday life, production, welding, private business and the automotive sector.
DOWNLOAD PRICE LIST for GAS WELDING EQUIPMENT
Acetylene cylinder, 40 l.
Acetylene cylinder, TU 6-21-40-85, 40 l., re-certified (re-examined)
Specifications
Volume, l - 40 Operating pressure, MPa - 9.8 Dimensions: Diameter, mm - 219 Height, mm - 1400 Material - Steel 45, L Cylinder weight, kg - 65 Type of shut-off device - valve GOST 9909-81.
Argon cylinder, 4 0 l .
Argon cylinder, GOST 949-73, 40 l., re-certified (re-examined)
Specifications
Volume, l - 40 Operating pressure, MPa - 14.7 Dimensions: Diameter, mm - 219 Height, mm - 1400 Material - Steel 45, D Weight of the cylinder, kg - 65 Type of shut-off device - valve GOST 9909-81.
Nitrogen cylinder, 40 l.
Nitrogen cylinder, GOST 949-73, 40 l., re-certified (re-certified)
Specifications
Cylinder diameter, mm 219. Capacity, l 40. Height, mm 1755. Cylinder weight, kg 93. Working pressure, MPa 19.6.
Oxygen bottle , 40 l.
Oxygen cylinder, GOST 949-73, 40 l., re-certified (re-certified)
Specifications
Volume, l - 40 Operating pressure, MPa - 14.7 Dimensions: Diameter, mm - 219 Height, mm - 1400 Material - Steel 45, D Weight of the cylinder, kg - 65 Type of shut-off device - valve GOST 9909-81.
Carbon dioxide cylinder, 40 l.
Carbon dioxide cylinder, GOST 949-73, 40 l., re-certified (re-examined)
Specifications
Volume, l - 40 Operating pressure, MPa - 14.7 Dimensions: Shell diameter, mm - 219 Height, mm - 1400 Material - Steel 45, D Cylinder weight, kg - 65 Type of shut-off device - GOST 9909-81 valve.
Propane cylinder, 50 l. (NEW)
Propane cylinder (GOST 15860-84), 50 l. (NEW)
Specifications
Volume, l - 50 Operating pressure, MPa - 1.6 Test pressure, MPa - 2.5 Overall dimensions: Shell diameter, mm - 299 Height, mm - 980 Wall thickness, mm - 3 Material - steel B St. 3 sp Weight empty cylinder, kg - 22 Mass of liquefied gas, kg - 21.2 Type of shut-off device - valve GOST 21804-94.
Last modification time 1441015554
tehnonova.ru
Oxygen storage and transportation
Technical and medical gaseous oxygen is produced in accordance with GOST 5583.
It is stored and transported in steel cylinders GOST 949 under a pressure of 15 MPa. Oxygen cylinders are painted blue with the inscription “OXYGEN” in black letters.
Liquid oxygen is produced in accordance with GOST 6331. O2 is in a liquid state only when received, stored and transported. For gas welding or gas cutting, it must be converted back into a gaseous state.
Prevalence in nature.
K. is the most common chemical. element on Earth: the content of chemically bound carbon in the hydrosphere is 85.82% (mainly in the form of water), in the earth’s crust - 49% by weight. More than 1,400 minerals are known that contain K. Among them, the predominant minerals are those formed by salts of oxygen-containing acids (the most important classes are natural carbonates, natural silicates, natural sulfates, natural phosphates), and rocks based on them (for example, limestone, marble ), as well as various natural oxides, natural hydroxides and rocks (eg basalt). Molecular carbon makes up 20.95% by volume (23.10% by mass) of the earth's atmosphere. K. atmosphere has a biological origin and is formed in green plants containing chlorophyll from water and carbon dioxide during photosynthesis. The amount of potassium released by plants compensates for the amount of potassium consumed in the processes of decay, combustion, and respiration. K. - a biogenic element - is part of the most important classes of natural organics. compounds (proteins, fats, nucleic acids, carbohydrates, etc.) and in the composition of inorganic. skeletal connections.
Characteristics of oxygen
O2 characteristics are presented in the tables below:
Conversion coefficient for volume and mass of O2 at T=15°C and P=0.1 MPa
| Weight, kg | Volume | |
| Gas, m3 | Liquid, l | |
| 1,337 | 1 | 1,172 |
| 1,141 | 0,853 | 1 |
| 1 | 0,748 | 0,876 |
Conversion factors for volume and mass of O2 at T=0°C and P=0.1 MPa
| Weight, kg | Volume | |
| Gas, m3 | Liquid, l | |
| 1,429 | 1 | 1,252 |
| 1,141 | 0,799 | 1 |
| 1 | 0,700 | 0,876 |
Oxygen in a cylinder
| Name | Cylinder volume, l | Mass of gas in the cylinder, kg | Gas volume (m3) at T=15°C, P=0.1 MPa |
| O2 | 40 | 8,42 | 6,3 |
Thanks to this table, you can now easily answer questions that welders often ask:
- How much oxygen is in the cylinder per m3? Answer: in a 40 liter cylinder there are 6.3 m3
- How much oxygen is in the cylinder? Answer: in a 40 liter cylinder there are 6.3 m3 or 8.42 kg
- How much does an oxygen cylinder weigh? Answer: 58.5 kg - the mass of an empty carbon steel cylinder according to GOST 949; 8.42 - kg mass of oxygen in the cylinder; Total: 58.5 + 8.42 = 69.92 kg weight of the oxygen cylinder.
In order to approximately find out how much oxygen is in the cylinder, you need to multiply the capacity of the cylinder (m3) by the pressure (MPa). For example, if the capacity of the cylinder is 40 liters (0.04 m3), and the gas pressure is 15 MPa, then the volume of oxygen in the cylinder is 0.04 × 15 = 6 m3.
Carbon dioxide cylinders 5l 10l 20l 40l 50l GOST 949-73
Carbon dioxide cylinders, small and medium volume, made of carbon and alloy steel GOST 949-73. (CO2 cylinder)
The body of the carbon dioxide cylinder is painted with black enamel, the inscription “ CARBON ACID ” is yellow. The weight of carbon dioxide cylinders is indicated without valves, caps, rings and shoes. Approximate weight: metal cap - 1.8 kg; rings - 0.3 kg; shoe - 5.2 kg
| Working pressure, MPa (kgf/cm2) | Carbon dioxide cylinders 50 liters | Carbon dioxide cylinders 40 liters | Carbon dioxide cylinders 20 liters | ||||||||||||
| Steel 45, D | Steel 30HGSA | Steel 45, D | Steel 30HGSA | Steel 45, D | |||||||||||
| Diameter, mm | Length, mm | Weight, kg | Diameter, mm | Length, mm | Weight, kg | Diameter, mm | Length, mm | Weight, kg | Diameter, mm | Length, mm | Weight, kg | Diameter, mm | Length, mm | Weight, kg | |
| 14,7 (150) | 219 | 1685 | 71,3 | 219 | 1660 | 62,5 | 219 | 1370 | 58,5 | 219 | 1350 | 51,5 | 219 | 740 | 32,3 |
| 19,6 (200) | 219 | 1755 | 93,0 | 219 | 1660 | 62,5 | 219 | 1430 | 76,5 | 219 | 1350 | 51,5 | 219 | 770 | 42,0 |
| Working pressure, MPa (kgf/cm2) | Diameter, mm | 12 liter carbon dioxide cylinders | 10 liter carbon dioxide cylinders | 8 liter carbon dioxide cylinders | 5 liter carbon dioxide cylinders | 4 liter carbon dioxide cylinders | 2 liter carbon dioxide cylinders | ||||||
| Steel 45, D | Steel 45, D | Steel 45, D | Steel 45, D | Steel 45, D | Steel 45, D | ||||||||
| Length, mm | Weight, kg | Length, mm | Weight, kg | Length, mm | Weight, kg | Length, mm | Weight, kg | Length, mm | Weight, kg | Length, mm / diameter, mm | Weight, kg | ||
| 14,7 (150) | 140 | 1020 | 17,6 | 865 | 13,0 | 710 | 12,4 | 475 | 8,5 | 400 | 7,3 | 330/108 | 3,7 |
Small volume cylinders can be supplied with a flat bottom. Do you want to buy carbon dioxide cylinders?
| CALL: | (8442) 780-530 |
| (812) 309-73-72 | |
| WRITE |
We will do the rest ourselves. We will deliver to a transport company or bring you to your city.
prom-kapital.ru