When two dissimilar metals come into contact and an electrolyte such as moisture is present, there is a possibility of bimetallic corrosion on the more electronegative or anodic metal, as defined in the electrochemical series, which corrodes first, preventing the other metal from corroding.
The bimetallic effect is the basis for the protection that zinc coating (hot-dip galvanizing) provides to small areas of exposed steel if the coating is damaged. Zinc coatings corrode first, protecting the metal below it in the electrochemical series. The degree of bimetallic corrosion will depend on a number of factors such as the metals in contact, the ratio of the areas of the contacting metals and operating conditions. Generally, the level of bimetallic corrosion will increase as the potential difference between the two metals increases, such as how far apart the two metals are in the galvanic voltage series. However, the potential may vary due to the formation of the oxide layer and cannot be used to determine the extent of bimetallic corrosion, as other factors listed below are also important. The area ratio of the contacting metals is essential, and ideally the anode-cathode metal ratio should be high. If the ratio decreases, problems may arise due to high levels of oxygen reduction, which can lead to increased corrosion of the anode metal. The influencing conditions are of great importance, because For bimetallic corrosion, the electrolyte must bind the two metals present. As a result, in dry environments (indoors) the likelihood of bimetallic corrosion is very low, while in outdoor atmospheric conditions the likelihood increases due to the presence of moisture in the form of rain and condensation. The worst conditions are immersion in a solution where the electrolyte permanently bonds the two metals. Typically, any possibility of bimetallic corrosion can be mitigated by electrically isolating the two metals from each other. For bolted connections, connections can be achieved by using neoprene or plastic washers, while for overlapped surfaces this can be achieved by using plastic spacers or painting one of the surfaces with a suitable paint coating system. Generally, hot-dip galvanized steel performs well in contact with most common structural metals when exposed to atmospheric conditions, providing a high galvanized steel to other metal area ratio. Conversely, under immersion conditions the effect of bimetallic corrosion is greatly increased and insulation is usually required.
Example of unacceptable galvanic pairs:
Galvanic action can occur if a stainless steel building structure is fastened with galvanized bolts. In this unwanted pair, the high anode fastener will suffer as its electrons move towards the cathode stainless steel. Therefore, fasteners must be made of a less galvanically active metal than the metal structure material.
The rate of galvanic corrosion is influenced by the surface area of the anode and cathode. If a large anode is connected to a small cathode, the anode will rust slowly, but if the opposite is done, it will rust quickly. For example, use stainless steel bolts to fasten aluminum, but not vice versa.
The intensity of contact corrosion also depends on the operating conditions of the joint. Under normal atmospheric conditions, the process will proceed less quickly and increases in an aggressive electrically conductive environment, for example, solutions of acids and alkalis. The presence of other substances in water increases the conductivity of the electrolyte and the rate of corrosion. Therefore, environmental assessment is important when designing structures.
Physical properties of stainless steels and compatibility with other materials
Compatibility with other materials
In practical applications, there is often a need to combine stainless steel with various metal materials in one assembly. When these materials are conductively connected to each other in a conductive environment, corrosion reactions occur that can cause damage due to contact corrosion.
According to DIN 50 900 part 1 - “contact corrosion is accelerated corrosion of the metallic region, which is caused by a corrosion element consisting of a metal/metal or metal/electron-conducting solid pair. In this case, the rapidly corroding metal region is the anode of the corrosion element.” The corrosion phenomenon that appears during contact corrosion is uniform or uneven surface removal of the layer. The surface removal of the layer or the loss of mass of the “ignoble” partner in this combination depends on the size of the fluid current of the elements (“potential differential current”) and the degree of intrinsic corrosion in the established mixed potential of the metal combination. The current of the elements is a complex quantity that depends on the geometric location, the size of the electrode surfaces coming into contact with the medium, the resting potentials and polarization resistances of the partners, as well as on the resistance of the electrolyte of the medium.
To assess the corrosion hazard of a non-noble partner in a combination of materials, what is important is not the value of the potential difference (voltage difference) between the materials connected together, but the characteristic of the potential curve of the partial current density of both materials in a corroded environment. The corrosive current density (element current) and thus the effect of contact corrosion can, at the same potential difference, vary by several orders of magnitude depending on the characteristics of the anodic and cathodic partial current density potential curve. The decisive factor is whether the anodic or cathodic partial reactions can occur unimpeded or with impediments, for example due to the formation of coating layers. If, with good conductivity of the corrosive medium, there are unfavorable relative areas (large cathode/small anode), then contact corrosion can cause corrosion damage.
Therefore, the use of a theoretical voltage series, but also a practical voltage series, is not suitable in practice for assessing the risk to materials of conductive contact. To accurately assess the hazards of material combinations, corrosion studies according to DIN 50 919 .
Physical properties
The physical properties of some selected steel grades are listed for comparison in the following table. The higher thermal expansion and lower thermal conductivity of austenitic steel should be taken into account. Due to the content of alloying components, its electrical resistance is higher than that of unalloyed steel.
An important difference between ferritic/martensitic chromium steel and chromium-nickel steel is magnetic susceptibility. Unlike magnetizable chrome steel, austenitic steel exhibits significant non-magnetizability properties in the diffusion annealed state.
Cold working under pressure can cause a change in structure in austenitic steel, so that limited magnetizability then appears. The nickel content significantly influences the magnetizability of austenitic stainless steel, so that by increasing the nickel content, the tendency to magnetization can also be significantly avoided in the cold-worked state.
Approximate values of tensioning moments and pre-tensioning forces for screws made of rust- and acid-resistant steel - A2/A4
Stainless steel corrosion
Anti-corrosion resistance
A fundamental prerequisite for achieving optimal corrosion resistance is a completely metallic clean surface. Stainless steel is characterized by special resistance to active chemical and water environments. They have a total mass fraction of the element chromium (Cr) of at least 12% and a mass fraction of the element carbon (C) of maximum 1.2%.
The high anti-corrosion resistance of stainless steel is based on its ability to form a so-called passive layer on the surface. In this case, we are talking about a layer of metal oxide or hydroxide only a few angstroms thick, which separates the metal from the exposed environment. The passive layer of stainless steel is not something permanent, but over time it is balanced in its composition and structure with the environment. After mechanical damage to the metal surface, a new passive layer is formed, generally independently.
If a sufficient passive layer cannot be formed in the medium, or if the existing passive layer is chemically breached or completely destroyed, corrosion damage may occur.
The alloying element that is decisive for the ability to form a passive layer is chromium.
Thanks to the increase in chromium content, as well as molybdenum (Mo) and also thanks to other alloying elements, resistance to significantly more aggressive operating conditions is increased.
Only the content of alloying elements soluble in the metal is effective for passivation. Therefore, maximum anti-corrosion resistance has a segregation-free matrix, which is depleted not only by precipitation or the formation of non-metallic phases, for example, chromium or molybdenum.
Stainless steel can have surface stripping corrosion and various forms of localized corrosion. Corrosion that removes a layer of metal appears primarily upon contact with acids and strong alkalis. But for practice, various forms of local corrosion are more important.
Intergranular corrosion
Intergranular corrosion is an effect along the so-called core boundaries, while the grains themselves are almost not destroyed or are slightly destroyed.
The impact on grain boundaries can go so far as to cause individual grains to separate from the grain structure, causing the structure to lose its cohesion. The cause of intergranular corrosion in stainless steel is the precipitation of chromium-rich carbides at the grain boundaries, which cause chromium depletion in the border zones. The chromium-poor zones formed in this way do not have anti-corrosion resistance to most attack agents and can therefore quickly dissolve.
Precipitation of chromium carbides requires a certain carbon content and occurs in a temperature range between approx. 500°C and 800°C, for example in heat treatment or welding processes.
To prevent chromium carbide precipitation, you can lower the carbon content of stainless steel to below 0.03% or bind the existing carbon using so-called stabilizing elements, such as titanium (Ti) or niobium (Nb), which have a greater chemical affinity for carbon than chrome.
If chromium carbide deposits have appeared, they can be dissolved again at diffusion annealing temperatures above 1050° C. In unstabilized ferritic steel, the existing tendency to intergranular corrosion can be eliminated by annealing at 800° C - 885° C. In this case, due to the additional diffusion of chromium chromium depletion in the zones bordering the grain is eliminated from the grain.
Penetrating and crevice corrosion
Penetrating and crevice corrosion is caused in practice in most cases by chromium ions. Along with this, the less common halides bromide and iodide may be the cause.
Perforation corrosion is caused by the interaction between halide ions and the passive layer, which breaks through locally. Sieve-shaped depressions are created and, due to their expansion, places of through corrosion, which can have different shapes. The danger of through corrosion increases with increasing concentration of halide ions, increasing temperature and increasing electrochemical potential of the steel.
Crevice corrosion occurs in crevices in which the exchange of fluid with the environment is limited. Such gaps depend on the design and operation and are located, for example, on flanges, in places where pipes are rolled, under gaskets, screw heads or also under the crust.
This corrosion mechanism corresponds essentially to the through-corrosion mechanism. Additional influencing factors are also the geometry of the gap and the type of gap-forming materials. Since crevice corrosion occurs even at a significantly lower corrosion load than penetrating corrosion, the appearance of crevice in chloride-containing media should be avoided as much as possible by design measures.
With a homogeneous distribution of alloying elements, the relative resistance to through and crevice corrosion of stainless steel can be determined by the active sum “W” W = % Cr + 3.3 x % Mo + 30 x % N oder W = % CR + 3.3 x % Mo .
The influence of the alloying element nitrogen is more complex than this ratio. The high efficiency expressed in the factor 30 can be fully manifested only in high-alloy steel with a high molybdenum content. Non-metallic contaminants, primarily sulfide deposits, contribute to penetrating and crevice corrosion if they reach the surface.
The advantages are the most smooth surface, which makes it difficult for sediments to adhere, which can cause crevice corrosion.
High resistance to through and crevice corrosion is achieved only with impeccable surface characteristics, i.e. with a metallic shiny surface. Therefore, tarnish and scale residues after welding, extraneous rust, grinding residues, etc. should be thoroughly removed.
Foreign rust
By extraneous rust we mean deposits of rust particles that do not appear in the appropriate place, but are transferred from somewhere outside. Foreign rust occurs mainly when “black” and “white” steel are stored and processed separately. But also abrasion of the tool can cause foreign rust. Due to the deposition of foreign rust, the conditions for crevice corrosion may be met.
Corrosion cracking
Media with specifically active components - especially chloride ions - can, when simultaneously exposed to tensile stress, cause a corrosive effect when a crack forms in stainless steel, even if the steel is sufficiently resistant without mechanical stress in the environment. This phenomenon, which is called corrosion cracking, can be caused not only by externally introduced tensile stresses due to operation.
Often the cause also largely lies in the inherent stress that occurs during processing, for example during welding, grinding or cold working.
The risk of chloride-induced corrosion cracking increases with both penetrating and crevice corrosion and with increasing temperature and chloride concentration. In addition to this, there are other factors at play on the material side. For example, austenitic steel types 18/10 – CrNi and 18/10/2 – CrNi-Mo are at risk of chloride-induced corrosion cracking at temperatures above approx. 50°C. But by increasing the content of molybdenum and especially nickel, durability can be significantly increased.
Also ferritic and ferritic-austenitic, stainless steel is comparatively less sensitive.
Vibration corrosion cracking
The resistance to vibration of all types of stainless steel is reduced more or less strongly due to additional chemical exposure. The decrease in vibration resistance depends on the means of influence and the multiaxiality of the emerging variable loads.
Contact corrosion
The possibility of contact corrosion exists when two metals with different free corrosion potentials are connected to each other by wiring in a corrosive environment. A metal with a lower free corrosion potential may be polarized to higher potentials and therefore subject to increased attack.
Also, if there is a large difference between the free corrosion potentials of the metals involved, corrosion does not necessarily occur. This depends on the electrochemical characteristics of both metals.
The conductivity of the medium and the characteristics of the metals involved are also important. If the “less noble” metal occupies a significantly larger surface area than the “more noble” one, and the corrosive environment is highly conductive, the risk of corrosion damage is less. But you should still avoid contact between the “base” metal with a small surface and the “more noble” metal with a large surface.
Stainless steel has generally high free corrosion potentials and is therefore not at risk of increased attack from contact corrosion. A much more common case is when contact corrosion occurs in other metals with a lower free corrosion potential due to contact with stainless steel.
Did you like the material?
comments powered by HyperComments
How to protect a structure or assembly from contact corrosion?
If for design reasons it is not possible to avoid unwanted contact of dissimilar metals, then attempts can be made to reduce galvanic corrosion using the following methods:
- painting surfaces in the area of their junction;
- application of compatible metal coatings;
- isolation of the connection from the external environment;
- electrical insulation;
- installation of non-metallic gaskets, inserts, washers in bolted joints.
Practice shows that in cases where the requirements for the admissibility of contacts of different metals are neglected, one has to pay dearly for this. Incorrect arrangement of contact pairs damages fastening units and metal structures and can cost human life.
«>
Copper and brass
If the installation requires contact between galvanized materials and copper or brass in a damp or humid environment, rapid corrosion of the zinc may occur. Even wastewater can contain enough dissolved copper to cause rapid corrosion.
If the use of copper or brass in contact with galvanized coatings is unavoidable, precautions must be taken to prevent electrical contact between the two metals. The connector surface must be insulated with non-conductive pads; connections must be made with insulating fasteners and a sealing sleeve. This is to ensure that the water does not re-spread and the flow of water from the galvanized surface to the copper or brass is not reversed.
Electroplating equipment
There is no need to “take away bread” from the owners of professional beauty salons. The corresponding techniques must be performed especially carefully so as not to cause harm to health. However, any ordinary person is able to prepare a high-quality set of equipment to solve technical problems.
The main component is a reliable and fairly powerful DC source. Adjustments in the desired range of voltage (1-12.5 volts) and current (up to 50-60 A) with a built-in indicator of the measuring device will be useful. The values of the required electrical parameters are selected after determining the operating settings of the technological operations.
A container with suitable dimensions is selected from a chemically neutral material. Heat-resistant plastic will do. However, it is better to use glass taking into account the following advantages:
- long-term preservation of consumer properties;
- strength, resistance to high temperatures;
- easy to clean.
Equipment set
As you can see in the photo, the electrodes can be fixed to the walls. The use of "crocodiles" speeds up the connection. To heat to the desired temperature, an electric stove with smooth power adjustment is useful. Scales are needed to accurately prepare the mixture.
Galvanic corrosion of aluminum
The most common design errors in aluminum structures are related to galvanic corrosion. Galvanic or electrochemical corrosion occurs when two dissimilar metals form an electrical circuit that is completed by a liquid or film electrolyte or corrosive medium. Under these conditions, the potential difference between dissimilar metals creates an electric current passing through the electrolyte, which (current) leads to corrosion primarily of the anode or the less noble metal of this pair.
The essence of galvanic corrosion
When two different metals are in direct contact with an electrically conductive liquid, experience shows that one of them can corrode, that is, become corroded. This is called galvanic corrosion.
The other metal will not corrode; on the contrary, it will be protected from this type of corrosion.
This type of corrosion is different from the types of corrosion that would occur if both of these metals were placed separately in the same liquid. Galvanic corrosion can happen to any metal as soon as two different metals are in contact in an electrically conductive liquid.
Appearance of galvanic corrosion
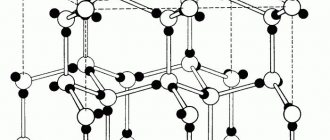
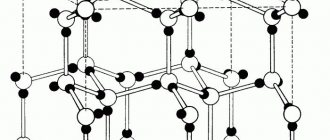
The appearance of galvanic corrosion is very characteristic. This corrosion does not spread over the entire surface of the product, as is the case with pitting corrosion. Galvanic corrosion is tightly localized in the area of contact between aluminum and another metal. The corrosive effect on aluminum is uniform; it develops deeper in the form of craters, which have a more or less rounded shape [3[.
All aluminum alloys are subject to identical galvanic corrosion [3].
Main types of aluminum corrosion
The following main types are typical for aluminum corrosion [4]:
- General corrosion
- Crevice corrosion
- Frettinig corrosion
- Stress Corrosion
- Galvanic corrosion
- Pitting corrosion
- Intergranular corrosion
- Subsurface corrosion
Figure 2 – General corrosion of aluminum: dissolution of the natural oxide film with solutions of strong alkalis and some acids [4]
Figure 3 – Crevice corrosion of aluminum [4]
Figure 4 – Fretting corrosion of aluminum: mutual friction of two aluminum components under rough contact conditions [4]
Figure 5 – Corrosion of aluminum alloys under stress: under certain conditions in Al-Cu, Al-Mg, Al-Zn-Mg alloys [4]
Figure 6 – Galvanic corrosion of an aluminum alloy occurs under conditions of its wet or moist contact with another, more “noble” metal, such as copper [4]
Figure 7 – Pitting corrosion of aluminum under the influence of chloride ions [4]
Figure 8 – Intergranular corrosion and subsurface corrosion [4]
Depending on environmental conditions, loading and functional purpose of the part, any type of corrosion can cause premature failure. In addition, improper use of aluminum parts and products can aggravate corrosion processes.
Distinctive features of copper
Copper can conduct heat six times more than regular iron. Because of this, welding must be performed with increased thermal energy, and in some cases even preheating of the base metal is possible.
Under normal conditions, copper is inert, but when heated it reacts with oxygen, hydrogen, phosphorus and sulfur. Oxygen is capable of oxidizing copper at high temperatures, and above 900 °C the oxidation rate increases significantly. This is due to the fact that the initial composition of copper contains oxygen in a bound state. Cuprous oxide forms a eutectic with a lower melting point (1065 °C). The melting point of copper is 1085 oC. Therefore, the oxygen contained in it worsens its positive indicators.
Difficulties in welding copper to stainless steel
The presence of hydrogen and its release into the atmosphere has an impact on the final result of welding with stainless steel. It can cause porosity in the copper and subsequently cause a crack in the weld. The solubility of hydrogen depends on the temperature and partial pressure in the protective gas atmosphere. During crystallization, hydrogen dissolves in copper twice as fast as in other iron.
During the welding process, there is a possibility of porosity appearing in the heat-affected area due to the accumulation of hydrogen there. Therefore, strict requirements are imposed on the metal being welded regarding its hydrogen content. Electroslag remelting and vacuum melting make it possible to reduce the hydrogen content in copper.
Sulfur in copper is present up to 0.1%, soluble in liquid form, but insoluble in solid copper. It does not have a significant effect on the quality of welding.
Due to the properties listed above, there are certain difficulties in welding copper with stainless steel:
- Different chemical composition . Hydrogen and oxygen present in copper can significantly reduce the quality of the weld.
- Different thermal conductivity coefficients (for stainless steel it is much lower).
- Different melting temperature regimes : stainless steel melts at 1800 °C, and copper at 1085 °C, actively reacting with atmospheric gases.
- The coefficient of copper dissolution in stainless steel has a maximum of 0.4%.
- During the formation of a weld seam, a sharp boundary is formed between steel and copper due to the oversaturation of steel inclusions.
- There is a possibility that a layer with microcracks will form in the steel, which will be filled with copper . To avoid this, it is necessary to move the welding arc slightly onto the copper part: in this way, molten copper is supplied to the weld area.
A reliable and durable weld can be obtained using manual argon arc welding. By fusing copper metal onto stainless steel using fluxes in the field of protective gases, the resulting connection will be resistant to long-term static loads (without losing its ductility). Before starting welding, it is necessary to treat the edges of the seam with a 10% caustic soda solution.
It is easier to weld stainless steel with pure copper than with additional inclusions. Such a composition without impurities is less common, so the choice of welding method and the basic technology of the welding process are the same as for other non-ferrous metals.
Galvanoplasty, galvanostegy, patination
Galvanoplasty is called copying technology. The essence of the processes does not differ from the above descriptions. However, adhesion is reduced to facilitate separation of the finished product from the workpiece.
Electroplating is an improvement in the mechanical parameters of the combined layer. Chrome, for example, prevents damage to steel products due to its high strength.
Patination is used to change the decorative properties of the surface. In particular, they create an artificially aged appearance.
Arrows mark areas created using the “rainbow” patination technology
Advantages and development history
Electrochemical activity series of metals
This technology was invented in 1838 by a scientist named Boris Jacobi. It was he who began the active introduction of galvanics into a variety of production processes. Soon, galvanic processing was successfully mastered by mints, artists, craftsmen, and industrial enterprises.
However, this technique received its name in honor of the Italian scientist Luigi Galvani. He began studying electrochemical processing technology almost simultaneously with Boris Jacobi.
The main advantages of galvanization include the following:
- Electroplated coatings are characterized by uniform thickness and the highest level of density.
- Electroplating can be easily applied even to structures with complex shapes.
- The coating resulting from galvanic treatment has good adhesion to many metals.
- The decorative and protective properties of galvanized parts are very high.
- The thickness of the galvanic coating is very easy to adjust.
By the way, the word “electroplating” is found not only in industrial fields and jewelry production, but also in cosmetology. This is the name of the process in which the skin is exposed to low-power currents to get rid of excess fat from the sebaceous glands.
Conclusion
The zinc layer applied by hot-dip galvanizing can maintain its performance properties for up to 120 years when used under normal conditions. This is due to the thickness of the zinc layer, which is up to 200 microns. As a result, the metal acquires high protective properties and is resistant to mechanical stress. Moreover, the coating is capable of self-recovery when cracks form, which is due to the special composition of the zinc solution.
In turn, the thickness of the zinc layer during galvanization is no more than 15 microns. Therefore, the service life of products with such a coating thickness in aggressive conditions can last no more than 1 year. The advantages of this technique are affordable cost, evenness and uniformity of the coating.
Conditions for galvanic corrosion
Galvanic corrosion is based on the same principle and in order for it to occur, the following three conditions must be simultaneously met [3]:
- various types of metals;
- presence of electrolyte;
- electrical contact between two metals.
Various types of metals
For any metals that belong to various types, galvanic corrosion is possible. The metal with the electronegative potential (or the more electronegative metal if they are both electronegative) acts as the anode.
The tendency of various metals to form galvanic couples and the direction of electrochemical action in various corrosive environments (sea water, tropical climates, industrial atmospheres, etc.) are shown in the so-called galvanic series. The further apart the metals in these rows are, the more severe galvanic corrosion can be. In different corrosive environments, these sequences of metals may be different (Figure 10).
Presence of electrolyte
The contact area must be wetted with an aqueous solution to ensure ionic conductivity. Otherwise there is no possibility of galvanic corrosion.
Electrical contact between metals
Electrical contact between metals can occur either by direct contact between two metals, or through a fastening connection, such as a bolt.
Figure 10 [1]
As can be seen from the graphs in Figure 10, aluminum and its alloys become anodes in galvanic cells with most metals, and aluminum corrodes, as they say, sacrificially and protects the other metal of the galvanic pair from corrosion.
Only magnesium and zinc, including galvanized steel, are more anodic and therefore, being exposed to corrosion themselves, protect aluminum from it.
Aluminum and cadmium generally have almost the same electrode potentials and therefore neither aluminum nor cadmium are subject to galvanic corrosion. Unfortunately, cadmium is recognized as very toxic and is used less and less, and in many countries it is simply banned as an anti-corrosion protection.
Materials and equipment used
Structure and properties of metals
The exception is cold galvanizing performed by Galvonol. This is a liquid suspension that is directly applied to the metal. It is unstable to some solvents and therefore requires a topcoat.
There are several groups of galvanic baths:
- Large ones. Designed for large-sized products.
- Average. They cannot accommodate a large product. At the same time, they remain the most in demand in conditions of medium-scale production.
- Small ones. They can only galvanize small parts.
Anode plates are placed in the bath. They are made from different materials. Their main task is to replenish the lost metal from the product during the galvanization process.
Important components are the type of electrolyte and current density. These parameters vary depending on the type of operation.
The compositions of cyanide baths for silver plating are presented in the table.
| Compound | Electrolyte number | |||
| 1 | 2 | 3 | 4 | |
| Cyanide silver | 2 | 6 | 30 | 100 |
| Sodium cyanide | 70 | 70 | — | — |
| Potassium cyanide | — | — | 70 | 100 |
| Sodium carbonate | 10 | 10 | — | — |
| Potash | — | — | 10 | 25 |
| Sodium hyposulfite | — | — | 0,4 | 0,5 |
| Ammonia aqueous, ml/l | — | — | 1-2 | 2 |
| Caustic potassium | — | — | — | 15 |
The current density influences the structure of the formed deposit. It is measured as the ratio of current per unit surface of the workpiece.
A little history
Luigi Galvani (1737-1798), whose name is given to the method of depositing parts of one metal onto the surface of another - electroplating - had nothing to do with the silvering of teaspoons and the galvanizing of aluminum buckets. He devoted himself entirely to theology, anatomy, physiology and physics, and was an outstanding physician of his time, who wanted to understand and explain the principles of “animal electricity”, when muscle contractions can be observed when current is passed through obsolete flesh. He was the first to describe the difference in potentials of different types of metals and electrolytes that arose during his experiments when they came into contact. He described and moved further in his electrophysiological research.
It occurred to the Russified German Moritz Hermann (1801-1874) to apply this difference for practical purposes. Having moved to Russia for permanent residence, Moritz changed his name to Boris, leaving behind the second of the ancient surnames that belonged to his family - Jacobi.
At the Imperial Academy of Sciences they laughed at Hermann a little, calling him “As if Boris” behind his back, but he also enjoyed enormous respect. It is he, a brilliant physicist and inventor, to whom Russia owes many brilliant inventions, who in 1840 wrote a work entitled: “A method for producing copper products using electricity using given samples from copper solutions or Electroplating for applied arts.”
It was with such a device that the history of the now widely used electrochemical process began, which has become a whole separate branch of modern industry.