History of the use of aluminum in everyday life
While the Germans cooked on enamel cookware, the British used these cookware to disinfect homes and hospitals. Napoleon Bonaparte in France required employees to serve food in aluminum plates, which were more expensive than gold plates. The mined metal sold for six hundred dollars a pound, and in the 1820s European nobility were willing to replace some gold and silver cutlery with aluminum.
However, aluminum quickly lost its shine. Mining of the metal brought the price down to $2.25 pounds in 1890. Despite the low price, housewives were not yet aware of the benefits of cooking in aluminum cookware.
On February 23, 1886, 22-year-old inventor Charles Martin Hall was experimenting with aluminum in a laboratory in Oberlin, Ohio. Hall's notebook notes indicate that he had perfected a procedure for inexpensively producing an aluminum compound that could be used in cookware.
Hall's efforts met with formidable resistance. Housewives did not want to give up their trusted tin products. The country's major department stores refused to stock the new product, whose benefits sounded too fantastic to be true. The turning point came in the spring of 1903.
A well-known store in Philadelphia gave a public demonstration of cooking in aluminum cookware. Hundreds of women watched in amazement as a professional chef prepared apple jam. As soon as spectators were allowed to step forward and make sure that the ingredients were not sticking to the pan, orders for aluminum cookware immediately began pouring in.
By the time Hall died in 1914, his fortune was worth $30 million, and he had spawned a new aluminum cookware that transformed the American kitchen.
Main applications of aluminum
This structural metal is widely used. In particular, it was with its use that aircraft manufacturing, rocket science, the food industry and tableware manufacturing began their work. Thanks to its properties, aluminum allows for improved maneuverability of ships due to its lower weight.
Separately, it is worth mentioning the ability of metal to conduct current. This feature made it the main competitor of copper. It is actively used in the production of microcircuits and in the field of microelectronics in general.
The most popular areas of use include:
- Aircraft manufacturing: pumps, engines, housings and other elements;
- Rocket science: as a combustible component for rocket fuel;
- Shipbuilding: hulls and deck superstructures;
- Electronics: wires, cables, rectifiers;
- Defense production: machine guns, tanks, aircraft, various installations;
- Construction: stairs, frames, finishing;
- Railway area: tanks for petroleum products, parts, frames for cars;
- Automotive industry: bumpers, radiators;
- Everyday life: foil, dishes, mirrors, small appliances.
Its wide distribution is explained by the advantages of the metal, but it also has a significant drawback - low strength. To minimize it, copper and magnesium are added to the metal.
Microscopic structure
The characteristic properties of metals can be understood based on their internal structure. All of them have a weak connection of electrons of the outer energy level (in other words, valence electrons) with the nucleus. The resulting potential difference in the conductor leads to an avalanche-like movement of electrons (the so-called conduction electrons) in the crystal lattice. The combination of these electrons is often called an electron gas. In addition to electrons, phonons (lattice vibrations) contribute to thermal conduction. Plasticity is due to a small energy barrier to the movement of dislocations and displacement of crystallographic planes. Hardness can be explained by a large number of structural defects (interstitial atoms, vacancies, etc.).
Due to the small return of electrons, oxidation of the metal is possible, which can lead to corrosion and further deterioration of properties. The ability of metals to oxidize can be obtained from the standard activity range of metals. This fact confirms the need to use metals in combination with other elements (an alloy, the most important element of which is steel), their alloys and the use of various coatings.
For a more correct description of the electronic properties of metals, quantum mechanics should be used. In all solids with sufficient symmetry, the energy levels of the electrons of individual atoms overlap and form allowed bands, and the band formed by valence electrons is called the valence band. The weak connection of valence electrons in metals leads to the fact that the valence band in metals is very wide and not all valence electrons are sufficient to completely fill it.
The main characteristic of such a partially filled zone is that even at a minimal applied voltage, the reconstruction of valence electrons begins in the sample, i.e. flowing electric current.
The same high electron mobility leads to high thermal conductivity and the ability to reflect electromagnetic radiation (which gives metals their characteristic shine).
The effect of aluminum on the human body
Aluminum in medicine
Despite the fact that aluminum in large quantities is harmful to human health, it is widely used in the treatment of a number of diseases.
Aluminum-based preparations are made that have enveloping, analgesic, adsorbent and antacid effects. The antacid properties of aluminum are used to reduce the acidity of gastric juice, since it very actively binds to hydrochloric acid. The indication in this case may be, for example, gastritis with high acidity (hyperacid gastritis). Aluminum preparations find both internal and external use.
The role of aluminum in the human body.
Aluminum plays a very important role - it takes part in the process of regeneration (restoration) of epithelial and connective tissues, maintaining bone strength, and in the formation of peptides and phosphate complexes. Aluminum affects the function of the parathyroid glands and has both an activating and an inhibitory effect on digestive enzymes. A person consumes aluminum with such products as: oat flakes, rye grains, wheat grains, flour, peas,
rice cereal, potatoes, kiwi, cabbage, carrots, apples.
Lack of aluminum in the human body
Microelement deficiency in the body is such a rare phenomenon that the likelihood of its development is reduced to zero.
Every year the amount of aluminum in the human diet is rapidly increasing.
The compound comes in food, water, food additives (sulfates), medications, and sometimes in the air. In medical practice throughout history, several isolated cases of deficiency of the substance in the human body have been recorded. Thus, the pressing problem of the 21st century is the oversaturation of the daily menu with an element rather than the development of its insufficiency.
Despite this, let’s look at the consequences of aluminum deficiency in the body.
- General weakness, loss of strength in the limbs.
- Slowing growth and development of children and adolescents.
- Impaired coordination of movements.
- Destruction of cells, tissues and loss of their functionality.
These deviations occur if a person regularly does not receive the daily requirement of aluminum (30-50 micrograms). The poorer the diet and the lower the intake of the compound, the more intense the symptoms and consequences of deficiency.
Excess aluminum is hazardous to health
Excess microelement is toxic.
An increased aluminum content is dangerous for human health, since immunity is reduced, and sometimes irreversible changes occur in the body, which sharply reduce life expectancy.
Characteristic signs of an excess of microelement: decreased hemoglobin, decreased number of red blood cells in the blood, cough, loss of appetite, constipation, nervousness, mental disorders, speech disorders, spatial orientation, clouding of mind. memory loss, convulsions.
Remember, aluminum belongs to the category of immunotoxic microelements, so to maintain health you need to monitor the amount of the compound entering the body daily.
If aluminum is considered an immunotoxic element for the human body, we decided to find out how aluminum enters the human body.
History of the use of iron in everyday life
Iron is a metal whose use in industry and everyday life has virtually no limits . The share of iron in global metal production is about 95%. Its use, like any other material, is determined by certain properties.
Iron played a huge role in the development of human civilization. Primitive man began to use iron tools several millennia BC.
Iron has not lost its importance to this day. This is the most important metal of modern technology. Due to its low strength, iron is practically not used in its pure form.
Iron-based materials are created that can withstand high and low temperatures, vacuum and high pressures. They successfully withstand aggressive environments, alternating voltage, etc.
The production of iron and its alloys is constantly growing. These materials are universal, technologically advanced, accessible and cheap in bulk.
The most common iron alloys are steel and cast iron.
Stainless steel is a very popular material for household items, with uses spanning everything from boilers to televisions. This is a very popular material for microwave ovens and is usually made from doors and interior panels and then coated with a light acrylic enamel to provide better visibility. In recent years, it has become quite popular to use stainless steel kitchen appliances as opposed to plain white. We are talking about refrigerators, freezers, dishwashers, ovens, stoves, as well as kettles and toasters.
Cast iron is a strong but non-ductile metal . Plumbing equipment (bathtubs, pipes, sinks, kitchen sinks), dishes, stairs, fences, and home furnishings are made from it.
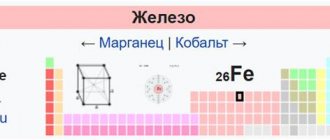
Position in Mendeleev's periodic table. Structure of the iron atom
Name: Iron (ferrum) Serial number: 26 Group: VIII Period: 4
Atomic mass: 55.847 Electronegativity: 1.83 Characteristic oxidation states: +2,+3
The iron atom consists of a positively charged nucleus (+26), inside of which there are 26 protons and 30 neutrons, and 26 electrons move around in four orbits.
The distribution of electrons among orbitals is as follows: 1s22s22p63s23p63d64s2.
Finding metals in nature
Metals with low chemical activity (Cu, Ag, Au, Pt, Hg) are found as free inclusions in rocks. Most metals occur in nature in the form of ores and compounds. They form oxides, sulfides, carbonates and other chemicals. In order to extract pure metals and use them further, it is necessary to separate and purify them from their ores. Perform alloys and other metal processing as necessary. This is studied by the science of metallurgy, which distinguishes between ferrous (iron-based) and non-ferrous ores (they contain no iron, about 70 elements in total). The exception is about 16 elements: the so-called precious metals (gold, silver, etc.) and some others (for example, mercury, copper), which are present without impurities.
They are also present in small quantities in seawater (1.05% - 0.12%), plants and living organisms (which play an important role).
Thus, the content of some metals in the earth’s crust is as follows: aluminum - 8.2%, iron - 4.1%, calcium - 4.1%, sodium - 2.3%, magnesium - 2.3%, potassium - 2, 1%, titanium - 0.56%.
Metals found in nature:
- in its original state: silver, gold, platinum, copper, sometimes mercury.
- in the form of oxides: magnetite Fe3O4, hematite Fe2O3, etc.
- in the form of mixed oxides: Kaolin АІ2О3 – 2SiO2 – 2H2O, alunite (Na,K)2O – АІО3 – 2SiO2, etc.
- various salts:
- Sulfides: PbS galena, NgS cinnabar,
- Chlorides: sylvinite KS1, NaCl-halogenite, sylvinite KSl-NaCl, carnallite KSl - MgSl2 - 6H2O,
- Sulfates: barite Vaso4, anhydride Ca8O4
- Phosphate: apatite Ca3(RO4)2,
- Carbonates: chalk, marble SASO3, magnesite MgSO3.
Most aluminum is concentrated in aluminosilicates, of which feldspars are the most common. Their most important representatives are the orthoclase minerals K [AlSi3O10], albite Na [AlSi3O10] and anorite Ca [Al2Si2O10]. Minerals of the mica group are very common, for example, muscovite Kal2[AlSi3O10][OH]2 and nepheline (Na, K)2[Al2Si2O8] (used for the production of alumina, soda products and cement). Among other minerals, the most commonly used are bauxite Al2O3*nH2O and cryolite Na3AlF6. A common product of rock destruction is kaolin, which consists mainly of the clay mineral kaolinite Al2O3*2SiO2*2H2O.
Most calcium occurs naturally as deposits of limestone and chalk, consisting primarily of the calcite mineral CaCO3 and marble. Among other rocks, the most common are dolomite CaCO3*MgCO3, anhydrite CaSO4 and gypsum CaSO4*2H2O, fluorite CaF2 and apatite 3Ca3(PO4)2*Ca(F, Cl)2. Calcium is found in significant quantities in various silicates, such as CfO*3MgO*4SiO2 (asbestos) and aluminosilicates.
Magnesium in nature is often found in the form of magnesite MgCO3 and dolomite, silicate Mg2SiO4 (olivine), kainite KCl*MgSO4*3H2O and carnallite KCl*MgCl2*6H2O. Natural compounds of alkali metals are NaCl*KCl-Sylvinite, NaCl-Halogenite and Na2SO4*10H2O-Mirabilite.
Iron is the most common metal in the world after aluminum. This is a component of numerous minerals that form accumulations of iron ores: Hematite Fe2O3, magnetite Fe3O4, hydrogeetite HFeO2*nH2O, siderite FeCO3 and others.
From time to time, local iron of meteorite or terrestrial origin is also found.
Many metals often accompany the most important natural minerals: scandium is a component of tin, tungsten and cadmium as an impurity in zinc ores, niobium and tantalum in tin ores. Iron ores are always accompanied by manganese, nickel, cobalt, molybdenum, titanium, germanium, and vanadium.
Main applications of iron
Iron often means not a substance as such, but low-carbon electrical steel - this is the name of the metal alloy according to GOST. Truly pure iron is not easy to obtain, and it is used exclusively for the production of magnetic materials.
Iron is ferromagnetic, meaning it becomes magnetized in the presence of a magnetic field. However, this property is highly dependent on the impurities and structure of the metal. The magnetic properties of absolute pure iron are 100–200 times higher than those of commercial steel. The same can be said about the grain size: the larger the grain, the better the magnetic properties of the substance. Mechanical processing also matters, although its influence is not so impressive. Only such iron is used to produce all magnetic materials for electrical engineering and magnetic drives.
Connections
All metals used in production are divided into non-ferrous and ferrous. Ferrous are iron alloys, in particular steel and cast iron, the rest - copper, nickel, silver - are non-ferrous. Ferrous metallurgy accounts for 95% of all metallurgical processes. Ferrous alloys are divided in this way:
Steel is an alloy of iron with carbon and other ingredients, whose mass fraction does not exceed 2.14%. Carbon gives steel its ductility and hardness. The composition may also include manganese, phosphorus, sulfur, and so on; Stainless steel is used in construction and mechanical engineering where greater than normal corrosion resistance is required; heat-resistant alloys “work” in conditions of high temperatures – turbines, heating lines. Heat-resistant - do not oxidize at high temperatures, which is important for many working units in heating engineering.
Cast iron is an alloy with carbon, where a higher content of the element is allowed - up to 4.3%. Moreover, cast irons differ in their properties depending on the form in which the alloy contains carbon: if the substance reacted with iron, white cast iron is obtained, if included in the form of graphite, gray cast iron is obtained; wear-resistant cast iron is used for the manufacture of pump parts, brakes, and clutch discs; heat-resistant is used in the construction of blast furnaces, open-hearth furnaces, thermal furnaces; heat-resistant is used in the construction of gas furnaces, in the manufacture of compressor equipment, and diesel engines.
The worst enemy of iron and its alloys is corrosion. A car has long ceased to be a luxury, but has become a means of transportation for most citizens. Now there are a huge number of cars from different manufacturers, different brands, but they are all made of iron-based alloys. We decided to find out which manufacturers' cars are less susceptible to corrosion and thus cause less trouble to their owners.
Magnesium alloy and nanoparticles for ultralight aircraft
The metal, developed on the basis of magnesium and silicon, took the best properties from its “parents”: density and lightness from magnesium, hardness from silicon. It was possible to combine these qualities in one material thanks to a special production technology - silicon carbide nanoparticles are not mixed with magnesium, but are sprayed into it. That is why the finished metal is strong and ductile, but at the same time resistant to high temperatures.
The researchers expect that their invention will find application in aircraft and automobile manufacturing; they also plan to use the material in the production of medical equipment and electronics.
This is what the surface of a new metal looks like under a microscope.
The effect of iron on the human body
The role of iron in the body
Iron has many functions. Here are the main ones:
Transport of oxygen to tissues . Iron is part of hemoglobin, the protein that makes up red blood cells (erythrocytes). It is iron that is responsible for the capture of oxygen, after which red blood cells transport it to all organs and systems of the body.
Metabolism . Iron in the human body is an integral part of many enzymes and proteins that are necessary for metabolic processes - the destruction and disposal of toxins, cholesterol metabolism, and the conversion of calories into energy. It also helps the body's immune system cope with aggressors.
A person consumes iron with foods such as: liver, shrimp, eggs, buckwheat, apples, nuts, cheese, beef, duck, wheat.
Iron deficiency
It is not surprising that iron deficiency affects appearance, health and well-being.
With a deficiency of this element, the skin becomes pale and dry, hair becomes dull and weak, and nails become brittle. Non-healing ulcers appear in the corners of the lips, and very painful cracks appear on the hands and feet. As the amount of iron in the body decreases, the state of health worsens - appetite disappears, many notice discomfort when swallowing. Sometimes tastes change in the strangest way, for example, a person really wants to chew chalk or chew paper.
People with iron deficiency experience constant loss of energy - they even wake up tired. The slightest physical exertion causes severe shortness of breath - this is due to the lack of oxygen. Other typical symptoms of iron deficiency are dizziness and even fainting, drowsiness, irritability, and memory impairment.
For people suffering from iron deficiency, constant colds and intestinal infections are typical. As we have already said, iron is directly involved in the work of the body’s defense system, and if it is deficient, the immune system cannot repel attacks of pathogenic bacteria in a timely manner.
These symptoms will probably seem very familiar to many. No wonder: according to WHO statistics, approximately 60% of the world's population has a lack of iron in the body, and in 30% the deficiency of this element is so great that we are talking about iron deficiency anemia - a condition in which the level of hemoglobin is significantly reduced.
Practical part: aluminum
Routes of entry of aluminum into the human body:
aluminum utensils and preparations containing aluminum ions
Study of the presence of aluminum ions in food prepared in aluminum cookware
Purpose: We often use aluminum cookware, so we decided to research the safety of aluminum cookware.
Experience No. 1
Aluminum reacts with water, releasing hydrogen and forming insoluble aluminum hydroxide. 2Al + 6H2O = 2 Al(OH)3 ↓ + 3H2↑
I boiled water in an aluminum pan for 15 minutes, then cooled the solution and checked its transparency.
Observations: No changes were observed in the water sample that was boiled in an aluminum vessel. By doing this experiment, I became convinced that the oxide film on the metal protects it from interaction with water, so aluminum oxide does not dissolve in water and does not react with it.
Experience No. 2
Aluminum reacts with alkali, forming salt and releasing hydrogen.
2Al + 2NaOH + 6H2O = 2Na[Al(OH)4] + 3H2
The oven and stove cleaner contains caustic soda, that is, sodium hydroxide. When applying this product to an aluminum object, I quickly saw signs of a reaction in the form of gas being released.
Conclusion: If aluminum reacts with alkalis, and in an alkaline environment it goes into solution in the form of salts, then cooking and storing food with an alkaline reaction of the environment will lead to the fact that it will also enter the food in the form of an ion.
Hydrophobic metal
Hydrophobic - water-repellent materials - are not uncommon today. However, in terms of their strength, all of them are unlikely to compare with the development of scientists from the University of Rochester. They managed to create a hydrophobic metal. For this purpose, the metal surface was treated with a special laser. The finest engraving gave the material new properties: it literally repels drops of water like rubber balls.
There are many areas where such material can be useful. This includes aircraft construction - hydrophobic metal will prevent aircraft from icing, and shipbuilding - airliner hulls will be less susceptible to corrosion.
Practical part: iron
Experiment “Study of iron content in apples.”
First I conduct an experiment on a red apple. I cut a red apple in half and examined the cross section of a red apple.
After some time, one of the halves of the apple, not smeared with lemon juice, darkened, and the one that was “protected” by lemon juice remained white.
I cut the lemon. I smeared one half of the apple with lemon juice, and placed the second half of the red apple cut side up on a plate. I did the same thing in the same sequence with a green apple. I put both halves of a green apple on a plate, cut side up, and began to observe the changes.
Conclusion. Darkening occurs due to the oxidation of iron, which is contained in apples, by atmospheric oxygen. The acid contained in lemon juice protects the apple cut from oxidation and slows down the oxidation process.
I noticed that the cuts of the red apple did not darken at all, which means that green apples contain more iron and are healthier.
Conclusion
Our life is unthinkable without metals. In our creative work dedicated to iron and aluminum, I expanded my knowledge about these elements, simple substances, metals, their properties and applications.
The role of iron and aluminum in the development and establishment of the technical culture of mankind is exceptionally great. Hardness, ductility, and malleability made them an indispensable material for the manufacture of tools and production. Looking out onto the street, we see hundreds of cars, each of which is made of iron. Cables, bridges, rails, trams, trains, and finally airplanes are made from iron or aluminum alloys. There are metals everywhere!... Well, we ourselves have these metals. They are used to carry out various processes in the body.
In our theoretical part, we characterized the structure and properties of iron and aluminum, their use in medicine, and described what happens when there is an excess or deficiency of ions of these elements.
As a result of the work done, we made the following conclusions:
Iron and aluminum ions have a vital effect on the human body.
Aluminum ions in large quantities are hazardous to health. Therefore, you should only cook in aluminum cookware and not store food. Do not get carried away with taking heartburn pills.
Iron ions have a beneficial effect on the human body. Therefore, it is necessary to eat foods rich in iron. Such as persimmons, apples, walnuts.
List of used literature
- Gorynin I.V. et al. Aluminum alloys. Application of aluminum alloys. Help Guide. Moscow "Metallurgy", 1978
- Hatch J. E. Aluminum. Properties and physical metallurgy. Directory. Moscow, "Metallurgy", 1989
- Rabinovich V. A., Khavin Z. Ya. Brief chemical reference book.
- Brief chemical encyclopedia. "Soviet Encyclopedia", 1963
- Karapetyants M.Kh., Drakin S.I. General and inorganic chemistry. "Chemistry", 1981
- Venetsky S. I. Stories about metals
- Beckert M.. Iron. Facts and legends.
Bibliography
- Erochin J.M. Chemistry. Textbook / J.M. Erochin. - M.: Publisher: Academy, 2003. - - 384 p.
- Ivanova R.G. Chemistry: Textbook for 10th grade of secondary schools. — 5th ed. / R.G. Ivanova, A.A. Kaverina - M.: Education, 2002. - 174 p.
- Korovin N.V. General chemistry / N.V. Korovin. - M.: Publishing house: Higher School, 2004. - 322 p.
- Reutov O.A. Organic chemistry. In 4 parts / O.A. Reutov, A.L. Kurtz, K.P. Butin - M.: Higher School, 2002. - 728 p.
- Savinkina M. A. Chemistry. Tenth grade. Textbook / M.A. Savinkina. — M.: Publisher: AST, 2003. — — 128 p.