Anodizing titanium black - Metalist's Handbook
The “ANODIZING TITANIUM” kit is used to form interference-colored anodic films on titanium and titanium alloys that retain the gloss of the original surface and have high light resistance, corrosion resistance and high anti-friction properties.
The kit "ANODIZING TITANIUM" includes all the necessary reagents used for preparing the surface and carrying out the process of anodic oxidation (anodizing) of titanium and titanium alloys. Using the “ANODIZING TITANIUM” kit allows you to obtain on a titanium surface, depending on the voltage in the bath, anode films of various colors and shades (brown, purple, blue, cyan, orange, yellow, turquoise, green, pink, crimson, etc. .). The differently colored sections of oxide films obtained on titanium alloys do not chemically interact with each other, have blurred color transitions and do not increase surface roughness.
Color anodizing of titanium and titanium alloys is used for marking products, for decorative finishing and giving titanium products various colors and shades, to increase corrosion, anti-friction properties, etc.
Process stages:
CHEMICAL DEGREASING → ETCHING → ACTIVATION → ANODIC OXIDATION
| ANODIZING TITANIUM AND TITANIUM ALLOYS | ||||
| ANODIZING COST | COST OF TITANIUM ANODIZING | |||
| Moscow, Saint Petersburg | 15 l kit | 30 l kit | 50 l kit | |
| 40-70 rub./dm2 | 40-70 rub./dm2 | ~ 10 rub./dm2 | ~ 7.5 rub./dm2 | ~ 6 rub./dm2 |
Using the “ANODIC ALUMINUM OXIDATION” kit for 15 liters, with periodic adjustments, you can anodize parts with an area of 30-35 square meters. meters
Need to purchase
- Current source (rectifier)
- Distilled or de-ionized water
Each conversion coating kit includes detailed process instructions. All chem.
The reagents included in the kit were pre-weighed and packaged in the required proportions.
All you need to do to prepare working solutions of electrolytes is to dissolve them in a certain sequence, according to the instructions, in distilled or demineralized water.
BRIEF TECHNICAL DATA:
Electrolyte temperature 15-25*C.
Current density 1-3 A/dm2. Voltage up to 120 V. The electrolyte is not adjusted during operation. Cathodes must be made of lead or corrosion-resistant steel. The ratio of anode to cathode surface area is 2:1. Titanium products are degreased with acetone or alcohol before the process.
The products are mounted on titanium hangers and secured with clamps or titanium bolts. Loading products into the anodizing bath is carried out at a minimum voltage, which is quickly raised, within 1 minute, to a voltage corresponding to the selected color. During the process, it is necessary to constantly stir the electrolyte.
Cost of kits
| CAT-05 | Set "ANODIZING TITANIUM" for 5 liters | 8000 R |
| KAT-15 | Set "ANODIZING TITANIUM" for 15 liters | 20500 R |
| CAT-30 | Set "ANODIZING TITANIUM" for 30 liters | 26800 R |
| KAT-50 | Kit "ANODIZING TITANIUM" for 50 liters | 33900 R |
The cost of individual chemicals reagents, accessories
| CAT-06 | Reagent “ORGAN SOLVENT” (1500 g) | 2100 R |
| CAT-07 | Reagent “EMULSITOR-S10” (200 g) | 450 R |
| KAT-08 | Reagent “ETCHING COMPOSITION” (10 l) | 1950 R |
| KAT-09 | Reagent “CLIGHTENING COMPOSITION” (3 l) | 1200 R |
| KAT-011 | Reagent “ANODIC OXIDANT No. 1” (1 kg) | 950 R |
| KAT-012 | Reagent “ANODIC OXIDANT No. 2” (3 l) | 700 R |
| KAT-SP | Lead counter electrode (250*360*2 mm); 2.02 kg | 1700 R |
Standard 'soft' or 'hard' packaging. Dispatch of the order within 4-8 business days by land or air. Some reagents require special packaging (due to reactivity or fear of cold). Order dispatch within 5-10 days by land or air transport
Reagents require special packaging (due to reactivity or fear of cold). Orders are dispatched within 8-14 business days by ground transport only.
Aluminum anodizing technology and advantages of the procedure
Aluminum is the best metal for making various parts. It is easy to process, the metal is light weight, high strength and does not corrode. But despite all the advantages, the appearance of this metal is not attractive.
Paints are very poorly retained on an aluminum surface, and if some kind of protective coating is not applied to the product, it will become covered with dark spots.
A technology such as aluminum anodization will protect the metal from oxidation and also give it an impressive appearance.
What is anodizing?
Anodization or anodic oxidation is a process that results in the formation of an oxide coating on the surface of the metal. The metal oxidizes.
The oxide film protects the metal surface from oxidative processes that occur when aluminum and air interact. When anodizing, the oxidized area is not removed, but a harder coating is formed.
The technology is similar to bluing.
Why anodize aluminum?
When this metal is in a natural environment, it combines with oxygen, and a protective film is formed on the surface. The protective layer prevents aluminum from oxidizing.
However, these natural oxides are very thin and can be easily damaged.
This problem can be solved by anodizing - this will improve the resistance of the metal to adverse external factors, as well as give it a more impressive appearance.
After the anodizing procedure, the metal is not at risk of corrosion. The protective film that forms on the metal during the anodizing process is highly resistant to wear. This coating will not peel off over time.
This coating is not the application of a protective layer, as is the case when coating steel with chromium or zinc. During the process of creating an anodized coating, the oxide film is formed directly from the metal itself. Not only aluminum can be anodized, but also other metals – titanium, magnesium.
Anodizing is often resorted to when it is necessary to enhance the decorative qualities of a given metal and give it a certain shade. Popular colors include light or dark gold, pearl color, and silver with a matte sheen. The colors of the coating can be changed; ordinary aniline dyes used for clothing are used for this.
In industrial conditions, anodizing technology is carried out in a 20% solution of sulfuric acid. However, anodizing aluminum at home using acid can be dangerous and very inconvenient. You wouldn't use this particular method, would you?
There is another technology, it involves the use of solutions of sodium carbonate and sodium chloride. These are soda and salt, which are found in every kitchen.
On video: how anodizing works.
Benefits of the procedure
There are several advantages that this technology provides:
- anodized aluminum profiles acquire significant protective properties;
- the metal surface is matte and uniform;
- the process allows you to eliminate damage to the surface - scratches, chips, stripes;
- the metal acquires high decorative properties;
- The thickness of the protective layer is quite large.
Warm anodizing
This technology is considered relatively simple. You can repeat it yourself. The process is carried out at room temperature. With simple manipulations you can obtain a beautiful colored coating using organic dyes. If you put in some effort, you can get several colors on the same part.
It is worth remembering the Soviet weapons - RPO-2, RPS-3, RPO-3. These guns were green, a color that is the result of anodizing the aluminum. The dye used was brilliant green, which is sold in every pharmacy.
The technology has advantages, but there are also disadvantages. Thus, anodized aluminum treated in this way does not have really high corrosion protection.
Corrosion occurs in sea water, as well as in places of contact with aggressive metals. Processing metal in this way also does not provide powerful mechanical protection - the surface is easily scratched by an ordinary needle.
If the technology is broken, then the coating is completely erased by hand.
This coating serves as the basis for painting. It's hard to imagine such high adhesion. If, after anodizing an aluminum profile, you paint it with epoxy paint, you will get a very reliable coating and aesthetics. Epoxy paint will stay on the surface for a very long time.
Warm anodizing is very simple. The first step is to degrease the parts and secure them in the suspension. Anodize until milky and wash the part with cold water. Paint in a hot dye solution and fix the painted surface for an hour.
Features of metal painting
A huge number of dyeing methods have expanded the color base.
Depending on the method of applying the paint and varnish coating and the oils, acids and varying degrees of heating used, it is possible to create any color, even lemon color. All paint application options are divided into two types:
- mechanical;
- chemical.
When applying paint mechanically, various powders are used, which are sprayed, splashed, hammered in using special tools. This option is not distinguished by quality, beauty or uniqueness, but is appreciated by car enthusiasts due to its low cost. In addition, mechanical painting can be easily done at any service station or even with your own hands if you have the necessary equipment.
The use of the chemical method requires experience and knowledge of the sequence of the entire procedure. The first thing to do is to strip the metal surface of the old paintwork. Next, the damaged areas of the primer are restored, which are previously cleaned of rust. After the metal acquires an ideal surface, it is degreased. Small oil removal parts are placed in solvents such as gasoline, ethyl or ether. The treated parts are immersed in boiling water and only then subjected to chemical staining.
Applying a paint coating is not enough if you want the metal to acquire an elegant look and shine of the surface and remain so even with frequent use. This problem is solved by oxidizing the steel.
This procedure involves rubbing the surface with mixtures such as:
- nitric acid mixed with alcohol, water and copper sulfate in the form of sawdust;
- inky-nut-colored acid, including iron and antimony;
- silver nitrate with added water;
- olive oil with antimony chloride.
After applying one of these mixtures to a metal surface, it is subjected to heat treatment. The result of the work will depend mainly on how well the part was cleaned from grease, dust and dirt.
This method is often used to protect weapons and car parts from scratches and chips. Protection can be achieved in other less effective but cheaper ways. For example, many people do metal bluing with oil at home, which, thanks to the crystalline structure of the protective layer of paint, penetrates and creates shine and an additional layer of protection.
If you are doing all the work yourself, then it is important to remember that after drying the painted metal will look darker than when wet. Therefore, taking into account such features, you need to adjust the color in an acceptable direction.
Metal oxidation methods
[Metal oxidation] at home allows you to solve two problems at the same time: renew the metal surface of any product and additionally protect it from corrosion.
Previously, it was believed that oxidation processing could only be performed in a production environment using industrial equipment, but human intelligence has proven that this is not the case.
The differences between processing metal products at home and in production lie in the difference in the technologies used, but they pursue the same goal.
As a result of the industrial oxidation process, a change in structure occurs in the upper layer of the metal.
At home, the steel surface is coated with a special substance that helps change the shade and protect it.
Features of the chemical process
Chemical treatment of a metal surface involves the use of solutions and melts of various oxidizing agents, for example, salts of chromic or nitric acid.
Their use makes it possible to provide anti-corrosion protection to the metal. In this case, processing can be carried out using both alkaline and acidic compounds.
The process of chemical oxidation by the alkaline method occurs at a temperature of 30-1800, which is determined by the type of metal.
For example, chemical oxidation of aluminum and its alloys is performed at a temperature of 80-1000, the processing time is 10-20 minutes.
The shade of the film formed on the surface of non-ferrous metal depends on the thickness and structure of the alloys.
If the chemical oxidation of aluminum is performed in an alkaline solution of low concentration and at low temperature, a thin protective film with a tarnished color can be obtained.
Conversely, if you make a too concentrated alkali solution for aluminum and its alloys and use a high processing temperature, the protective coating will be loose.
:
A long period of oxidation can result in etching of the metal.
Processing of complex alloyed stainless steel (steel oxidation) occurs through the use of a concentrated solution of nitric acid.
At a temperature of 18-550 with a duration of 15-60 minutes.
Features of anodic oxidation of metal
Anodic oxidation of metal products at home is performed using electrolyte compositions under the influence of direct current.
IMPORTANT TO KNOW: Technology for stamping sheet metal parts
In this case, the vessel in which anodic oxidation will be carried out should not be conductive.
Sulfuric acid (H2SO4) diluted with water can act as an electrolyte at the rate of 20% per 800 ml of water.
In this case, the acid is not diluted with water, but the water is diluted with acid. You can replace H2SO4 with baking soda and salt.
Using an aluminum hanger, the workpiece to be processed is attached to the anode, and a lead plate is attached to the cathode.
If the metal product has a complex shape, then more lead elements are used.
The distance between the plates and the product should not be more than 90 mm. The processing temperature should be 200, with a current density of 2-3 Amps per square dm.
The voltage at which anodizing will be carried out is 12-15V for 60 minutes.
Micro-arc oxidation is considered one of the anodizing technologies; the technical result of its use is to obtain a coating with pronounced decorative characteristics and higher protective ability.
:
Microarc oxidation gives the surface of non-ferrous metal uniformity, anti-corrosion resistance and microhardness.
The components of the composition are:
- water;
- H3BO3 (20-30 g/l);
- potassium alkali (4-6 g/l);
- starch (6-12 g/l).
Using the above list, you can make an electrolyte at home by regular mixing.
Next, microarc oxidation of aluminum alloys is performed in anode-cathode mode at a temperature of 25-300.
At a current density of 15-20 Amps per square dm, with a duration of 90-120 minutes.
Thermal oxidation of metals
Thermal oxidation of iron, alloys and stainless steel is a process that results in the formation of an oxide film layer on the surface of metal products.
Thermal oxidation is performed under high temperature conditions using steam or oxygen.
The equipment used to carry out thermal oxidation is a special furnace.
Therefore, it will not be possible to carry out heat treatment using this method at home.
The use of furnaces in oxidation technology eliminates the use of chemicals, etching, washing and a number of other processes.
IMPORTANT TO KNOW: Device for bending profile pipes
The processing temperature of metal products in thermal furnaces can range from 3500 to 7000, depending on the type of steel.
Technology of oxidation of copper and its alloys
Oxidation of copper is not difficult to perform using chemical and electrochemical methods, as a result of which the copper surface can acquire a variety of colored coatings.
To obtain copper film, cyanide or acidic liquid is used. Good performance is achieved by oxidizing copper with cyanide electrolyte.
At the same time, copper alloys, which contain alloying metals in their structure, are more difficult to process.
:
An example is bronze, which contains a certain percentage of tin, which helps protect copper from oxides.
Or an alloy of bronze with nickel and chromium additives, such a metal is even more difficult to process.
Bronze with a minimum presence of zinc, not exceeding 20%, lends itself well to processing, while a large amount of it complicates the process.
With the help of sulfur compounds, cold processing of copper sculptures is most often performed. As a rule, these are liver sulfur, ammonium sulphide and sodium.
Ammonium sulfide allows you to make a cold black with a blue tint oxide coating. You can give a decorative look to a product made of bronze and tin using sulfur liver.
But if you use it to color pure copper or bronze and tombac, you can achieve a red tint to the film layer.
Silver oxidation technology
Oxidizing silver allows the white metal to obtain a blue, black or purple tint, while the structure of the processed product is not subject to deformation or destruction.
You can treat silver items at home using liver sulfur.
To prepare the composition at home, you need to take potassium alkali and sulfur (you can buy it where fertilizers are sold).
Then you need to combine the substances in an iron container: 1 part alkali and 2 parts sulfur, and keep the composition on fire until completely melted.
The mixture must be stirred periodically. Next, the finished sulfur liver is removed from the heat and allowed to cool.
IMPORTANT TO KNOW: Equipment for aluminum casting at home
When the alloy has cooled, it is broken into pieces and transferred to a container with a tight lid.
Now that you have a sulfur liver at home, you can start processing silver. You need to take a piece of alloy, about the size of a pea, put it in a container and fill it with hot water.
:
After the lump is dissolved by stirring, the silver item is placed in sulfuric water.
After half an hour, the silver will begin to change its color, as mentioned above, the white metal can take on a purple, black or blue tint.
When the product acquires the desired color, it is removed from the liquid and rinsed with hot, warm and, finally, cold water.
Titanium oxidation technology
Oxidation of titanium is mandatory due to the low wear resistance of this type of metal.
Obtaining an oxide film allows titanium products to acquire chemical strength, increase the frictional characteristics of the material and change the color of the surface coating.
To carry out the oxidation of titanium, anodic treatment is most often used, since titanium does not withstand the effects of chemical solutions during the chemical oxidation process.
Anodic oxidation of titanium involves the use of oxalic, chromic and other acids or mixtures thereof, as well as other additives.
The black oxide film helps to strengthen the surface structure of titanium products; it is the result of using anodizing technology with an 18% sulfuric acid solution.
Depending on the processing mode, the protective film acquires a certain thickness.
:
For example, if the process is performed at a temperature of 800C, the anode current density is 0.5 Amperes with a processing time of 8 hours, the film layer will be about 2.5 microns.
When anodizing in the mode: 100ºС, duration – 2 hours, current density – 1 Ampere – the film thickness will be 1 micron.
Ways and methods
Cold method
According to the equation, the optimal temperature at which it is necessary to carry out anodizing processes using this technology is 0 °C. However, variations from –10 to +10 °C are permissible. It is at these temperatures that a strong and intact oxide film is formed on the surface of a titanium alloy part. The cold method allows you to carry out the procedure of solid anodic oxidation at home.
With proper adjustment of the current, sputtering can be carried out using electroplating using gold, copper or chromium as the material. Such a barrier coating will protect titanium products from oxides and rust, which extends its service life to several decades.
The main disadvantage of this anodizing technology is the impossibility of further painting the treated object.
Warm method
The technology involves the use of organic dyes, thanks to which the metal can be given an amazingly beautiful decorative look. Both ready-made coloring compositions and available dyes from a home medicine cabinet are suitable: iodine, brilliant green, potassium permanganate, iodinol, etc.
Unfortunately, this technology is not designed for hard anodizing. The barrier properties of the oxide film are very weak, as is the protection from mechanical damage. However, upon further painting, the oxide coating exhibits high adhesive abilities. Enamel paints adhere perfectly to such a coating, and in turn provide the titanium product with reliable protection against corrosion.
Online piercing store 69 LEVEL
Titanium in body piercing jewelry is commonly referred to as Ti6AL 4VEli Gr23 alloy with ASTM F-136 specification. Ti6AL 4VEli Gr23 alloy is the most common titanium alloy used in medicine, as well as in the military, space and chemical industries. Due to the wide range of applications of the alloy, different specifications have been developed for each of them.
The most common alloy specifications are developed by the American company ASTM, according to which most metallurgical factories operate. In addition, pure titanium Grade1 of ASTM F-67 specification, also used in medicine, is sometimes used for piercing.
Specifications ASTM F-136 and ASTM F-67 are standards that describe the chemical, mechanical and metallurgical requirements for the use of titanium products for implantation purposes without changing the chemical composition. Unscrupulous piercing manufacturers often replace titanium Ti6AL 4VEli Gr23 with a “dirtier” analogue Ti6AL 4V Gr5, in which the tolerances for impurities are greatly increased.
In the table you can see the chemical composition of titanium alloys, where Grade1 is pure titanium, Grade2 is its analogue with a large permissible amount of impurities.
| Chemical composition | ||||
| Chemical element | ASTM Grade | |||
| (Acceptable values) | 1 | 2 | 5 | 23 |
| N, Nitrogen | 0,03 | 0,03 | 0,05 | 0,03 |
| C, Carbon | 0,1 | 0,1 | 0,1 | 0,08 |
| H, Hydrogen | 0,015 | 0,015 | 0,0125 | 0,0125 |
| Fe, Iron | 0,2 | 0,3 | 0,4 | 0,25 |
| Oxygen | 0,18 | 0,25 | 0,20 | 0.13 |
| Al, Aluminum | 5,5-6,75 | 5,5-6.5 | ||
| V, Vanadium | 3,5-4,5 | 3,5-4,5 | ||
| Ti, Titanium | Bal. | Bal. | Bal. | Bal. |
What color should titanium be?
Titanium alloy can have various shades, from dark gray to almost silver (which in appearance is indistinguishable from surgical steel).
The color of a titanium alloy depends on the amount of iron impurity in it (for example, the Steel and Silver brand has an iron content of 0.042% with an allowable 0.25% in Gr23 and 0.4% in Gr5), which makes the alloy very light and ideal for anodizing, and , of course, depends on the quality of polishing (the better the polishing, the lighter the product).
If the iron content in the alloy is high (but still acceptable, for example 0.2%) - the piercing jewelry will have a dark tint and will anodize very poorly after about 65 volts (the jewelry will become dark gray matte with a pink or purple tint, to turquoise, and Moreover, such decoration will definitely not reach green).
What is titanium anodizing?
As you understood above, titanium can be anodized, that is, you can give titanium jewelry the color you want.
By passing a direct current of the required voltage through the titanium jewelry in distilled water (with a special anti-scale agent), a thin film of titanium oxide of the desired color is formed on it.
It makes the jewelry smoother at the micro level, which has a good effect on healing. Jewelry that has already been anodized, for example blue, can always be anodized again with a higher voltage, for example, gold.
On the contrary, it is impossible to make a blue tint out of gold. The service life of anodizing depends on the place where the jewelry is worn, for example, in the tongue it will begin to wear off after a few months, and in the earlobe anodizing will remain good for several years.
Why do you need mirror polishing for jewelry?
We all know that poorly polished jewelry can scratch the canal, and also secretions can accumulate in the irregularities of the jewelry, which are a breeding ground for bacteria, which increases the healing time of the puncture.
But the quality of polishing also greatly influences the occurrence of allergies to aluminum, vanadium and impurities contained in minimal quantities in ASTM F-136 titanium alloy.
With high-quality polishing of jewelry at the micro level, the surface of the jewelry becomes as smooth as possible, which makes it possible to form an oxide film that prevents the diffusion of titanium alloy atoms into the puncture.
What is black titanium?
It is impossible to anodize titanium jewelry black, but there are PVD and IP coatings. In terms of durability and appearance, these coatings are the same and it is impossible to determine on the finished product how it was applied. Only the coating technology differs.
PVD (Physical Vapor Deposition) is a method of applying a durable and wear-resistant layer in a vacuum by condensing vapor from particles of the substance selected for spraying. This results in the formation of a thin and very durable molecular film (PVD coating) with a thickness of 1 to 3 microns, due to which the jewelry becomes smooth and scratch-resistant.
IP (Ion plating) is the process of directing a stream of ions of the substance chosen for plating onto the metal, which form a very durable thin layer 1-2 microns thick, which makes the jewelry smooth and scratch-resistant.
To cover piercing jewelry, pure titanium (for spraying black, as well as all colors of anodized titanium), pure gold and zirconium, which are completely hypoallergenic, are used as sprayed material.
What does anodizing do?
Pothole repair of road surfaces: technology, methods, GOST
In some ways, anodizing is similar to the galvanic processes that occur during chrome plating or galvanizing of steel. But there is a significant difference: the use of foreign substances is excluded, even if they are similar in properties and chemical composition. Oxidation is carried out on the basis of the metal itself, which is subjected to electrochemical action.
When anodizing, the process can be regulated, the oxide layer is given predetermined properties, and the result is the strength of the oxidized area.
The best protective layer as a result of anodization is formed on metals such as aluminum, titanium, steel, tantalum. The main requirement for the technology is that the metal has only one oxide with high adhesive properties.
But to ensure adhesion, a porous structure is needed, which will ensure contact of the working mixture with the clean metal surface, which significantly speeds up the oxidation process.
- The first type is the porous surface of the oxide film. It is obtained when metal is exposed to acidic electrolytes. A surface structured with pores serves as an excellent basis for laying paint and varnish materials on it, which, with their structure formed during the polymerization of the base, is fixed in the fractals of the pores. That is, the anodized surface promotes increased adhesion.
- Barrier. Belongs to the second type. This is an independent protective coating that protects the metal from contact with an external aggressive environment.
However, the anodizing process is not limited to creating protective layers. By using different materials and changing the voltage level, you can get different shades of anodized film. What designers actively use when decorating interiors when aluminum is used as the facing material.
Titanium
golubka_mia
What is titanium?
Titanium (Ti - Titanium )
is an element of the secondary subgroup of the fourth group, the fourth period of the periodic table of chemical elements of D. I. Mendeleev. Titan was discovered in 1790. William Gregor, clergyman and amateur geologist, in Cornwall, England.
However, it was not refined until 1910, refined, or produced in commercial quantities until the early 50s. Since that time, titanium production has increased by about 8% per year and from the early 60s it began to be used not only in the military industry, but also for commercial purposes.
While pure titanium was prized for its combination of high strength, lightness and excellent durability, aerospace applications required even stronger materials.
In the 1950s, a high-strength alloy called 6-4 (6% aluminum, 4% vanadium, 90% titanium) was developed and immediately found use in the production of cars and aircraft frames.
Titanium is the most “elven” material, named after Titania, the queen of the elves from W. Shakespeare’s play “A Midsummer Night’s Dream.”
The name was given to the material for two reasons: because of its amazing lightness and because of the unusual shades of titanium oxide, which amazed scientists who first obtained titanium oxide in the laboratory.
There are three grades of titanium used in the medical industry: G1, G5 and
G23
.
Titanium G1
is designed for dental purposes. It has a low degree of polishing, which increases its implantability. That is, it grows into the body.
This property is successfully used for dental prosthetics, but makes it undesirable for jewelry for piercing and body modification. Titanium G23 is used for these decorations
. This material is also used to make pacemakers and other medical implants.
Titanium G23
has the highest degree of biocompatibility; a more biocompatible material has not yet been obtained.
Products made from Titanium G23 are 100% safe for health and are recommended by the International Piercers Association for installation in fresh punctures and accelerating the healing of problematic punctures.
Titanium G5 is a surgical alloy also used in transplantology. Although its purity and biocompatibility are lower than Titanium G23, it is optimal for piercing purposes. This is the golden mean in terms of price/quality ratio between Titanium G23 and Titanium G1.
If your body is not prone to the formation of keloid scars (otherwise, piercing is generally contraindicated for you), Titanium G5 jewelry will be quite sufficient even for a fresh piercing. In any case, the biocompatibility of Titanium G5 is higher than that of 585 gold or 316L surgical steel.
Titanium production is very high-tech and expensive. It is approximately 16 times more expensive than steel production. In addition, titanium alloys are difficult to machine. Therefore, real titanium jewelry cannot be cheap.
Anodizing, electrochemical oxidation - the formation of a protective oxide film on the surface of metal products.
Titanium anodizing not only creates a highly durable film on the metal surface, but also serves aesthetic purposes. Black titanium anodizing has a unique pearlescent hue, and colored titanium anodizing has bright, rich colors and can also be obtained with an iridescent iridescent finish.
Titanium anodizing is so strong that it cannot be scraped off even with a rasp. Titanium anodizing technology was created for the military and aerospace industries and is unmatched in its durability. So if you can't afford to buy titanium jewelry, you can buy cheaper and nicer titanium anodized pieces.
Another Zircon Gold coating is successfully used in the jewelry industry -
titanium anodizing with a gold effect without the participation of gold.
Such coating can be distinguished from gold only by sawing the product or carrying out an analysis in the laboratory. You can buy a cheaper (and more durable) product and successfully wear your “gold” without fear of it darkening, wearing off or causing an allergic reaction. Just don’t show anyone that there is no sample on it. golubka_mia
An old legend about a Broken Heart says. In ancient times, when human love was pure and considered sacred, two lovers lived in the world. The girl wanted to give her lover her heart so that it would never be separated from her beloved and went to the witch. The sorceress said: “I will make you a heart of crystal. It will always be with your lover, but if he cheats on you, your heart will break, and its fragments will scatter throughout the world.” The girl believed in the sincere and pure love of her lover and easily agreed to the witch’s conditions. Years passed. Love kept the lovers young and beautiful, but the witch grew old. She envied the girl's beauty, with the help of witchcraft she turned into her double and seduced her lover. The girl returned and found an ugly old witch in the arms of her lover. Her crystal heart broke at that very moment and scattered throughout the world in countless fragments, but the light of love in it did not go out. Until now, lovers find its fragments and Great Love is kindled in their hearts. Antique style ring and earrings. Metal - cupronickel. The coating is blackened silver. Inserts - red and white Swarovski crystals.
Attention! Limited quantity of goods.
Welcome to the mystical, mysterious world of the Lilith online store. We provide courier delivery throughout Russia, Ukraine and Belarus and postal delivery throughout Russia. You can go to the store product page by clicking on the picture.
Page 3
Titanium nitride coating. Application, properties, titanium nitride coating technology
Oxidation is a special type of procedure for coating a metal material with an oxide film. As a result of this process, a thin film appears on the metal surface, which performs a barrier function. It protects the material from air and moisture.
Oxidation of metal is one of the most effective methods for protecting it from the formation of rust on the surface. The film covers it with a fairly dense layer. After the procedure, all metal oxidation processes completely stop. As a result, products that are processed using the oxidation method last longer and retain their attractive external qualities for many years.
This processing procedure for various types of products is used not only to protect metal products from corrosion. This function is known to many. However, in some situations it is used to give a metal product decorative qualities.
Today, many types of metals undergo oxidation.
In this regard, the following are highlighted:
This procedure occurs quite often. It is used for:
As a result, after processing, the metal receives a small layer of oxide film, which has excellent protective qualities.
The procedure itself does not take much time. It is carried out after preliminary preparation of the metal. Its surface must be clean and degreased so that the oxide film has better adhesion to the aluminum.
For aluminum, another technology called color oxidation of aluminum is used. Due to this, a film of a certain color is formed on the surface of the metal. This process is decorative in nature. The effect of this method lasts for quite a long period of time.
Today, oxidation of steel products is not uncommon. They are susceptible to the formation of a corrosive film.
Chemical oxidation of steel
To process steel material, a chemical type of oxidation is used. It consists in immersing steel in a specially prepared acidic solution, which promotes the formation of an oxide film on the surface of the steel. It has a small thickness. However, it has a high level of durability.
Before the metal is treated with an oxidizing agent, it is carefully prepared. To do this, special products are used to remove dirt and greasy film.
As is known, metals such as titanium and its alloys have a low level of wear resistance. In order for the metal to acquire strength and hardness, various methods are used. One of them is oxidation. Thanks to it, a protective film appears on the surface of the metal, which increases the strength of titanium significantly.
Table 1. Metal oxidation - surface preparation
| Composition and mode | Solution number | ||
| 1 | 2 | 3 | |
| Composition, mass fraction, % | |||
| sulfuric acid (density 1.8 g/cm3) | — | 90—92 | 20—30 |
| nitric acid (density 1.4 g/cm3) | 95-97 | 5-6 | 40—60 |
| hydrofluoric acid or its salts | 3-5 | 0,5—1 | 10—12 |
| Operating temperature, K | 290—300 | 290—300 | 290—300 |
| Shutter speed, min | 0,1—0,2 | 1—2 | 0,2—0,3 |
"Blitz" master class on titanium anodizing
Good day everyone! Just now, in the topic of the respected AleksSi, I promised to find titanium and photograph the process of anodizing it in different colors, since people are interested, and I am an electrochemist at home and a chemist at work. Why the “blitz”? Because the purpose of the master class is to show the basics of this process. This is the first time I’ve done this myself, there may be inaccuracies on my part, please fill in! Of course, there are many subtleties: alloy grade, electrolyte, coverage area, current strength. Today I will look at the process handicraft, using the example of processing small areas by “drawing”.
Anodizing is the electrochemical deposition of thin oxide films. In titanium, these films are colored due to the interference of light. Everything is described in more detail on Wikipedia, etc., I won’t waste space. The colors depend on the voltage; there are approximate color schemes on the Internet.
I have a 10 volt offset from the majority. Color largely depends on the electrolyte and surface area. In the future, I hope to show you dip anodizing. I immediately apologize for the somewhat faded photos of the process, it’s a bit dark. At the end there are photos taken in bright daylight.
I wrote the text one at a time, sorry for any mistakes!
So. To work you will need titanium . I ordered a couple of sheets of OT4-0 and VT1-0 alloy from the guys from Hansa. The titanium content in OT4 is 95.938 - 99.6%, in VT1-0 - 98.61 - 99.7%.
When preparing the master class, I found information that anodizing colors vary depending on the brand - on pure titanium they are more faded. To be honest, I didn’t notice much of a difference.
I will draw on OT4-0, it is cleaner in appearance, the other sheet is dirtier and more scratched.
I also read that colors look more vibrant on a matte surface, which was confirmed. For matting I used a matte brush for a Dremel.
Before anodizing, thoroughly wipe the surface with acetone, otherwise your fingers will be visible.
Power unit. The color of the coating changes mainly in 10 Volt increments, I used a DC power supply at work to cover the range of colors that was most accessible to me.
The unit is old, older than me, but it honestly produces 99.9 Volts (and they cost a lot now). Colors are placed “on top”, you cannot change “70 Volt color” to “20 Volt”, vice versa - please.
Attention, remember that alternating current (and coating is possible with its help) is hazardous to health!
Anodizing titanium at home with your own hands
Translated by alexlevchenko92 for mozgochiny.ru
Anodizing titanium is an extremely interesting and useful activity that is very easy to do your own hands at home.
Anodizing is used in industry to improve the (corrosion) resistance of metals.
In addition, it is also used as a decorative decoration for jewelry (due to the wide range of colors obtained).
Step 1: Electrolysis
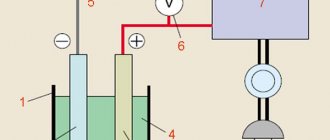
The first method of anodizing titanium is based on the principles of electrolysis. A piece of titanium will act as a positive anode, which should be immersed in a bath of electrolyte. (borax or sulfuric acid is usually used).
Step 2: Materials and Tools
- Aluminium foil;
- A plastic container large enough (to hold the piece of metal being anodized);
- From 1 to 8 9V batteries;
- 1.5 m of insulated wire;
- Sodium tetraborate (Borax);
- Hot water;
- Spoon;
- Latex gloves;
- Acetone or alcohol;
- A plastic cup.
Tools (optional):
- Ticks;
- Wire stripper.
Step 3: Prepare the electrolyte
Take borax and hot water, mix everything in a cup. Stir the solution until all the borax is dissolved.
Take aluminum foil and completely cover the plastic container with it. Wrap excess foil at the top edge of the container.
Step 4: Power Supply
Remove about a centimeter of insulation from both ends of the wire (60 cm long). After this, we will make a small hole in one of the corners of the aluminum foil. Pull one end of the wire into the hole and twist everything together.
Pour the prepared electrolyte into the container.
Let's take the required number of 9 V batteries and connect them as shown in the figure. Then we connect the wire that is attached to the foil to the negative terminal of the battery. Let's take another wire and connect it to the positive of the battery (this wire will be attached to a piece of titanium).
Step 5:
Different voltages will create different colors on the titanium surface. Please note that you can always change the color by increasing the voltage, but you will not be able to return to colors produced at a lower voltage.
The image shows the sulfuric acid anodizing process, but the results are very similar to borax anodizing. If you are not sure about the voltage value, you need to perform a series of experiments with a gradual increase in voltage value.
Step 6: Cleaning the Titanium
We will wear rubber gloves to prevent fingerprints from appearing on the surface of the titanium after cleaning. Then wipe the surface with alcohol.
Step 7:
Let's take a piece of titanium and attach a positive electrode to it. Let's immerse it in the solution. In this case, you should make sure that the wire does not touch the liquid and the titanium does not touch the foil. This may result in a short circuit (in case of contact).
Once the desired color is achieved, extract the titanium and dry it. You will see the color change due to your fingerprints.
This is completely normal (the original color can be restored with acetone or alcohol).
Step 8: Thermal Method
This method uses high heat, which will “thicken” the oxide layer on the surface of the titanium. I highly recommend using a blowtorch (although you can use a gas stove).
Step 9: Materials/Tools
- Heat source;
- Tongs or pliers (before using, make sure that the handles have reliable thermal insulation);
- Alcohol or acetone;
- A cup of very cold water.
Step 10:
Let's clean the titanium surface.
Step 11:
Take the cleaned titanium with tongs/pliers. Then turn on the stove/blowtorch and bring the piece of titanium close to the flame. You will notice that the color changes to yellow after about 30 seconds. By heating titanium for a longer period of time, you can achieve red, blue and a little green.
Step 12:
Once the desired color is achieved, turn off the heat source and immerse the metal in cold water.
How to care for titanium jewelry
Titanium jewelry is experiencing a new wave of popularity. There are many reasons for this: they are incredibly strong, durable and wear-resistant. And also bright: anodizing allows you to paint metal products in a wide variety of colors, which is atypical for the jewelry world.
But nothing lasts forever, not even titanium. Jewelry made from this metal can also lose its brightness, become scratched, or become damaged over time. In order to preserve their original appearance longer, it is important to provide proper care.
Clean regularly
You can use dishwashing liquid to clean titanium jewelry. You will need very little: a couple of drops per liter of water. It is better to choose a standard product without adding balm, cream or aggressive components. An ammonia cleaning solution can be used for the same purpose.
Cleaning is carried out according to the following scheme:
- Find a clean container and pour room temperature water into it. Add a couple of drops of detergent and stir. Whipping the foam is not necessary.
- Immerse titanium jewelry in the solution. It is advisable that the products are completely covered with water. Wait 5-6 minutes.
- Remove jewelry from the cleaning solution and rinse under running water. If this is not done, the products will remain sticky.
- Take tissue paper or soft cloth. Wipe the jewelry and check if there are any traces of dirt left on them.
Commercial products, such as special sprays, can also be used to clean titanium products. It is sprayed onto the metal surface, left for a couple of minutes, then wiped with a napkin.
Harsh cleaning products containing chlorine must not be used. This may damage the decoration.
Anodized titanium
When cleaning anodized titanium jewelry, you need to be especially careful. The algorithm is as follows:
- Soak the jewelry in the cleaning solution for a few minutes to remove dirt. But do not rub contaminated areas, otherwise you may disturb the color layer. Rinse with water.
- Soak jewelry items for a couple of minutes in an ammonia solution. Then blot very gently with a clean cloth, much like you would clean your glasses.
- Rinse the jewelry again with water and wipe dry.
If after cleaning at home the color layer is still damaged, contact a workshop that works with titanium jewelry. In most cases, the product can be anodized again.
Jewelry with stones
Choose a cleaning method that is suitable for your gemstones. Some of them do not like prolonged contact with moisture or detergents. Pearls, opal and turquoise are especially vulnerable.
If you are unsure how to clean a particular type of gemstone, be careful not to soak your jewelry in the cleaning solution for long periods of time. It is better to wipe the product with a soft damp cloth.
Polish
Non-anodized titanium jewelry can be polished using special polishes. This will help eliminate dull color, make scratches less noticeable, or get rid of them completely. It is prohibited to use polish for colored jewelry.
Another popular solution is titanium wax or varnish. It is applied in a small amount to the fabric and then rubbed into the product in a circular motion. Then wipe off the remaining product with a clean napkin.
Don't get dirty
In order for titanium jewelry to retain its original appearance longer, it must be removed every time you:
- wash the dishes;
- working in the garden;
- cook;
- doing the cleaning.
Contaminants on jewelry accumulate gradually. They “eat up” the shine and make the color of the metal less bright and saturated. To avoid having to thoroughly clean the product every time, you can use a soft cloth to remove light dirt.
Wear carefully
For your safety, never wear titanium rings while playing sports or working with electrical equipment or open machinery. This is not only harmful for the decoration, but also dangerous for its owner. If the titanium ring accidentally gets caught on a protruding element, there is a high risk of injury.
If the jewelry contains other metals in addition to titanium, such as gold or platinum, it should be removed before visiting the pool or shower. Chlorine contained in water can damage the product.
You will find more useful and interesting articles about jewelry and decorations on our website: DragZoloto.ru
Anodizing aluminum at a low price (anodic oxidation, aluminum oxidation)
You can order anodizing services. aluminum in our campaign. We guarantee the quality of the applied galvanic coatings on products. To clarify the cost of anodizing, contact our manager.
What is anodizing (anodic oxidation, an.ox.)
Anodization (oxidation) – electrochemical oxidation, the formation of a protective oxide film on the surface of metal products by electrolysis.
When anodizing, a product immersed in an electrolyte is connected to a positively charged electrode of a current source (anode).
A film with a thickness of 1 to 200 microns protects metal from corrosion, has electrical insulating properties and serves as a good basis for paint and varnish coatings.
Application of anodic oxidation of parts
Anodizing is used for decorative finishing of products made of aluminum and its alloys, enamel-like coatings on aluminum and some of its alloys, and is also used to protect magnesium alloys from corrosion, increase the antifriction properties of titanium alloys, and to coat electronic equipment parts made of niobium, tantalum, etc., in aircraft, rocket and instrument engineering, radio electronics.
Immediately after machining, aluminum reacts with oxygen in the air, so under normal conditions the surface is always covered with a thin oxide film. The structure of the film and its composition depend on the influence of atmospheric phenomena.
But aluminum always has an oxide film 2-3 nm thick. This film protects the metal from further oxidation and has excellent electrical conductivity.
The oxide film forms on pure aluminum at room temperature and has an amorphous structure (not crystalline) and is therefore not a good corrosion protection.
Aluminum protective coating
Aluminum is protected from corrosion by creating a crystalline oxide film 20-30 microns thick on its surface. In subsequent stages of the anodizing process, this film may be colored or may retain its natural color.
Anodizing aluminum also allows you to obtain various decorative effects, such as a mirror surface, matte and semi-matte surface, imitation of polished and ground stainless steel.
Aluminum anodizing process
Before starting the anodizing process, it is necessary to clean the aluminum surface from contaminants and remove the oxide film. To do this, degreasing and etching processes are carried out.
A process that results in the formation of highly porous aluminum oxide layers on the metal surface. The anodizing process is electrochemical.
There are two types of oxide films that are formed during the anodizing process:
Barrier - oxide film grows in neutral solutions in which aluminum oxide is difficult to dissolve. These are mainly ammonium borates, phosphates or tartrates.
Porous - oxide film grows in acidic electrolytes, in which the oxide can not only precipitate, but also dissolve. The most widely used is dilute sulfuric acid H2SO4. You can also use oxalic and phosphoric acids.
In the first seconds of anodization, a barrier layer forms on aluminum, first forming in the active centers on the surface of the metal. From these embryos, hemispherical lens-shaped microcells grow, which then grow together into a continuous barrier layer. When in contact with six surrounding cells, the shape of a hexagonal prism with a hemisphere at the base is formed.
Under the influence of local effects of electrolyte ions, pores appear in the barrier layer (in the center of the cells), the number of which is inversely proportional to the voltage.
In the pore, the thickness of the barrier layer decreases and, as a result, the electric field strength increases, while the ion current density increases along with the oxidation rate.
But, since the temperature in the pore channel also increases, which promotes etching of the pore, dynamic equilibrium sets in, and the thickness of the barrier layer remains practically unchanged.
This completes the anodizing process, and we obtain a coating with remarkable optical and technological properties.
Advantages of anodizing products
Anodized products can serve for decades without changing their decorative properties. Anodic corrosion protection is so effective that it can protect parts from the most aggressive influences. These remarkable properties have long been appreciated by car manufacturers, builders, military, and aircraft manufacturers.