Those who remember well the school curriculum for the Chemistry course will immediately answer the question of what electroplating is. For those who have forgotten a little, let us remind you that this is a branch of electrochemistry, the so-called process when a metal coating is applied to almost any product. This process is also used on an industrial scale, for example, both in the galvanizing or chrome plating of metal products, and in the manufacture of decorative items.
The process of deposition of electrolytes onto the desired surface is quite complex and requires compliance with safety precautions and certain home processing skills. Electroplating at home will not allow you to increase the strength of a metal product (this requires industrial capacity), but it can be used to decorate individual items.
Process Features
Electroplating, including at home, is very similar to electrolysis (where electricity is used to separate a chemical solution), which is the reverse procedure in which batteries produce electrical currents.
Electrolysis circuit
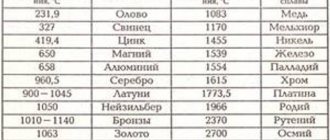
It is necessary to use the correct electrodes and electrolyte solution for electroplating at home, predetermining the chemical reaction or reactions that will occur when the electric current begins to act. The atoms that attach to the product come from the electrolyte. Therefore, if there is an electroplating process for copper plating, you need a copper electrolyte, and for gold plating, you need a gold-based electrolyte.
During electroplating, the master must make sure that the product to be used is completely clean. Otherwise, when atoms from the electrolyte arrive at it, they will not form a good bond, and the elements may simply precipitate. Typically, cleaning is accomplished by immersing the electrode in a strong acid or alkaline solution, or by (short) connecting the plating circuit in reverse. If the electrode is truly clean, the atoms from the metallization combine into a very strong crystalline structure.
Gilded items
Electroplating, done at home, involves passing an electric current through an electrolyte. This is done by dipping two terminals, called electrodes, into electrolyte and connecting them to a circuit with a battery or other power source. Electrodes and electrolyte are made from carefully selected elements or compounds. As electricity flows through the circuit, the electrolyte is broken down and some of the atoms of the material it contains are deposited in a thin layer on top of one of the electrodes. In this way, home electroplating is realized. All types of metals can be plated in this way, including gold, silver, tin, zinc, copper, cadmium, chromium, nickel, platinum and lead.
To achieve an even electroplating finish, the technician must first clean the surface of your metal object and prepare the necessary equipment. Dirt and oils on the surface may prevent the donor element from covering the surface. Start with degreasing, detergent (dish soap), and then scrub the metal with an abrasive acid cleaner to get a very clean surface.
A little chemistry
Cold galvanizing involves coating structures with a layer of zinc, which prevents the interaction of iron with atmospheric oxygen.
Zinc is a chemical element of the group of metals, silvery-white in color, quite fragile in its pure form.
As a result, zinc oxide is obtained on the contact surface, which is a very strong, practically insoluble chemical compound that covers the structure with a continuous film.
In addition, the electrochemical potential of zinc is almost half that of iron, so when paired with iron, zinc will be the anode, and iron will be the cathode.
And as a result of an electrochemical reaction in the presence of moisture from the atmosphere, pure zinc is converted into zinc carbonate.
Zinc carbonate, like its oxide, dissolves very slightly, covering the outer surface of the product with a stable film.
Video:
Thus, zinc provides two types of protection for metal structures at once: barrier and electrochemical.
A special feature of zinc coating is that if the layer is damaged, the resulting zinc carbonate “heals” the defect, instantly eliminating the likelihood of corrosion of the main structure.
Materials for technology
Necessary equipment if galvanoplasty is performed at home
- Metal object to be coated (must be steel).
- Power supply (3v-6v).
- Zinc sulfate / zinc hydroxide / zinc chloride.
- Water.
- A glass (instead of a glass or plastic object).
- Zinc (can be found inside Zn-C batteries).
- Sand paper (120).
- DIY galvanic bath or similar container.
- Tissue paper.
- Wires.
- A clean work area is sufficient for electroplating.
- A DC power source with voltage regulation is required; a household outlet is not suitable.
Galvanic installation
What do you need to prepare electrolyte at home? Different products require different solution compositions. For the solution, water with acids and other important inclusions of salts and metals is used. Do-it-yourself electroplating allows you to process many parts and tools for decoration or to increase wear resistance. The temperature of the electrolyte plays a different role in different operations. For example, when chrome plating, the higher the temperature, the brighter the coating is expressed.
How to prepare the product
Having collected the weight of the necessary components, prepared the containers, heating system and power source, we proceed to preparing the product that we want to process.
In order for the metal from the electrolyte to settle in an even layer on the object, it must be very well cleaned, otherwise the galvanic coating at home will turn out uneven and fragile. Some items will simply need to be degreased, others will require cleaning with sandpaper and grinding to remove corrosion and “burrs” from the surface.
Important! High-quality degreasing is provided by acetone solution, alcohol and even gasoline.
Steel products are kept for several minutes in a solution of sodium phosphate heated to 90 degrees. Non-ferrous metals are also degreased in sodium solution, but without heating.
Materials that cleaning chemicals cannot remove
There are some materials that chemicals cannot remove, or are difficult to remove, for the electroplating process. Here is a list of the most common of these materials:
- weld slag and other welding flux residues;
- splashing and splashing;
- burrs (may include excessively rough edges from flame cutting);
- mill coatings such as varnishes or varnishes found on some types of pipes;
- epoxy, vinyl and asphalt;
- sand and other impurities for castings;
- oil paints and markers;
- pencil markers;
- very heavy or thick deposits of wax or fat.
These materials must be removed from the surface before it is delivered to the galvanizing plant or in the case of domestic conditions.
Surface before and after cleaning and galvanizing
There are various generally accepted standards for abrasive blasting, hand cleaning and power tool cleaning that are effective in removing these materials. Abrasive blasting is usually necessary for castings to remove sand and other impurities from the casting process. Alternatively, a variety of products can be used that are compatible with the electroplating process to reduce the need for blasting or power tool cleaning. The use of uncoated electrodes avoids the problem of flux deposition during welding, which is harmful during the operation. Markers are available that easily dissolve in the baths used in the galvanizing process.
Safety precautions
Before you begin the galvanizing process, do not forget about safety precautions. Do-it-yourself electroplating does not involve manipulation, for example, in the kitchen. We are talking more about a garage or barn, a non-residential place with good ventilation, where grounding can be organized.
Important! Don't get poisoned by toxic fumes ! Galvanization can cause real harm to health. Organize an exhaust hood and cover your face with a respirator mask.
Thick rubber gloves are required. Protect your eyes with glasses. Before starting manipulations, read special literature. If you experience any symptoms of discomfort, consult a doctor immediately.
Electroplating at home with muric acid
To set up a home plating system, you will need water, muriatic acid, a 6-volt flashlight battery, a pair of wire clamps, a piece of copper, a piece of metal to process, and a container to hold the components used during liquid plating. The 6-volt battery has two terminals for easy connection to the system. It is acceptable to use a less powerful power source.
- Crocodiles fix a piece of copper (as a source of elemental ions that will be used for plating) and the main workpiece. Steel and nickel are two elements that can easily be plated with copper.
- After cleaning the surface of the material with various detergents, it is necessary to create a galvanic solution.
- 5 parts water are mixed with 1 part hydrochloric acid. Do not add water directly to the acid! Such actions cause a violent reaction with possible explosions.
- Always maintain a 5:1 ratio. For example, if you need more than 5 cups, measure 10 cups of water and add 2 cups of acid. Use plastic tools for mixing, as acid destroys metal. The top of the container will begin to heat up as the acid reacts with the water.
- Connect the alligator clip to the power source terminals. The battery will supply the current needed for the plating process. Attach one clip to one alligator clip and the other to the second terminal of the battery.
- Connect the copper to the positive terminal of the battery. Using an alligator attached to the positive terminal of the source, secure the other end with a metal piece of copper. In another scenario, galvanizing will not be able to work.
- Connect to the circuit the part that will be connected to the negative terminal of the battery. If possible, attach the clip in a place where galvanization is not necessary. If there is no free space to attach the clip, you will need to reposition the crocodile during the process to ensure that the product does not show clip marks and the coating is uniform throughout the entire area.
- If the process doesn't work, make sure you have the correct terminals installed.
- Immerse both elements in the prepared bath of dilute hydrochloric acid. The copper piece does not have to be completely immersed in the solution, but the product that is being processed is completely immersed in the working environment.
- To ensure an even layer, it is recommended to periodically stir the solution in the container.
- The two pieces need to be kept away from each other to avoid spots where copper accumulates too quickly.
- Using this method makes it difficult to get a thick layer of copper, but you can get a thin coating. When you are satisfied with the appearance of the material, the object is pulled out and dried.
Coating can take from a few minutes to several hours. After the desired layer has been formed, the material must be dried.
Main methods of galvanizing
Protective zinc coating is applied in two main ways.
- Hot. This is a factory method where the car body, after cleaning with acids and applying flux, is dipped into a bath of molten zinc. It is expensive and complicated, so it is not used by all automakers, even world-famous ones. This coating is the most durable and resistant to mechanical stress. It is available on cars from the brands Porsche, Volvo since 1975, Chevrolet Lachetti, some Ford and Opel models, as well as the VAZ 2110 (optional).
- Cold. In turn, it is divided into galvanic and mechanical. Galvanizing is also quite reliable, but the entire body is galvanized using this method only in factories, since it involves the use of large volumes of aggressive substances - alkalis, acids, ammonia. It is used by manufacturers of the brands Mercedes, Skoda, Volkswagen, Toyota, Reno Logan. In limited areas it can also be used for self-galvanizing. Mechanical methods of applying a protective coating (painting with compositions containing zinc) are the least reliable, but they are simple and cheap.
Electroplating with a metal ionic electrolyte solution at home
To electroplate at home with this method, you will need a piece of copper, the metal to be plated, vinegar, hydrogen peroxide, clamps, a 6-volt flashlight battery, a plastic container.
Use a container large enough to submerge the material you are trying to pour.
- Mix and heat equal parts vinegar and hydrogen peroxide. To make four cups of solution, add two cups of vinegar to two cups of hydrogen peroxide. The combination of vinegar and hydrogen peroxide makes peracetic acid, which should be handled with care.
- The copper pig should be dissolved in the composition. The liquid will turn blue, indicating that the solution contains copper ions, which can be used to plate the material.
- Soak the copper until the solution turns blue. It is better that the solution has a weak concentration; the solution should not be too dark.
- Attach the clamps to the battery. The battery provides the current needed to transport the metals from the donor to the recipient. Connect one alligator clip to the positive terminal of the battery and the other clip to the negative terminal.
- Clean metal at home to be electroplated. Before starting the galvanizing method, you must ensure that the metal is clean so that the new atoms can form a solid bond with the recipient metal.
- Connect the positive clamp to the copper part.
- Connect the negative alligator to the metal cover. Try to attach the alligator in an inconspicuous place. If you attach metal to the positive pole, electroplating will not work.
- Immerse the elements in copper liquid. Once both metals are connected, immerse them in the blue copper solution you prepared earlier. Since they are connected to the battery, current flows through the circuit. The procedure continues until a satisfactory level of coverage is achieved.
Everything you need to make electrolyte
Thermal thermodiffusion galvanizing method
The thermal method involves placing the element being processed in a bath of hot zinc solution, where, under the influence of temperature, a thin layer of protection is applied to the metal. Some car manufacturers practice applying zinc to the steel sheets from which the body is made, even during the rolling process. This method is slightly inferior in efficiency to the galvanic method, but also serves as excellent protection against rust. The Americans were the first to produce cars with hot-dip galvanized bodies, but after a few years the technology spread to Europe.
Features of galvanization with various metals at home
The option of applying a thin layer to a metal object at home can have a decorative function, or provide corrosion resistance to parts, and restore performance characteristics. Nickel plating is the process of depositing nickel onto a metal part. Decorative bright nickel is used in a wide range of applications. It provides high gloss, corrosion protection and wear resistance. In the automotive industry, bright nickel can be found on bumpers, rims, exhaust pipes and trim. It is also used for bright work on bicycles and motorcycles.
Nickel plating at home
A chrome layer at home can be decorative, provide corrosion resistance, facilitate cleaning procedures, or increase surface hardness. Sometimes a less expensive chrome simulant may be used for aesthetic purposes. Electroplating chrome plating at home can also be done at home.
Do-it-yourself copper plating
Copper plating is practiced to produce a protective layer or increase the electrical conductivity of the material. To create such a layer, poisonous cyanides, which are dangerous to life, are used. This operation is not performed at home. Initially, steel products are nickel-plated and only then coated with copper.
Galvanizing at home
Galvanizing is considered the simplest method of galvanizing products. The electrolyte consists of zinc sulfate (200 g), ammonium sulfate (50 g), sodium acetate (15 g) per 1 liter of water. In such a solution, the zinc will dissolve and then successfully coat the workpiece.
Product coating after brass plating
Brass plating is used for decorative purposes for fittings. For the operation, the electrolyte must contain copper and zinc salts mixed in a cyanide solution. Electroplating with brass at home is also not recommended.
Silvering and gilding have found industrial use as a conductor and decorative layer. The product is pre-plated with nickel and then coated with silver or gold. To carry out the operation, the electrolyte must contain silver chloride, ferric potassium cyanide, and soda ash. Such a liquid should be heated to 20 degrees, where graphite material can be used as an anode.
Silvering
Electroforming at home can be used to create exact replicas of metal parts, plates or circuits. Also, the use of technology will enhance the working properties of the workpiece. For such purposes, gold, silver, nickel, chromium or similar metals are used.
What are the advantages of cold galvanizing
- Simplicity.
You can easily carry out galvanizing yourself, even if you have never done it before. In addition, the solution dries very quickly - in most cases half an hour is enough. - Versatility.
Cold galvanizing is used to process hard-to-reach parts. In addition, the solution can be applied in any way convenient for you: roller, brush, spray gun. Some small parts can even be immersed in a zinc-containing solution. In addition, the material interacts with any solvent (solvent, xylene). Remember that this is the method that can be used to process wet metal. - Durability.
Cold galvanizing lasts about three times longer than heat treatment.
The essence and purpose of silvering
Silvering of metals, produced under any conditions, has not only decorative, but also practical purposes:
- silver has high hardness (up to 90 kg/mm²);
- conducts electricity well;
- resistant to the formation of oxides and rust.
The advantages of metal also include thermal conductivity and light reflection.
Silver is resistant to alkaline solutions and acids of organic origin. Even strong concentrations of sulfuric acid can only dissolve silver plating at 100 °C.
In a word, silvering of metals prevents premature destruction of the material, and under a layer of silver, alloys are less susceptible to various environmental influences.
Galvanic method
Galvanic galvanization involves placing it (or a separate element of it) in a container with a certain electrolyte. The container body is connected to the positive electrode of the power source, and the element being processed is connected to the negative electrode. Without diving deeply into electrochemical processes, the technology can be described in simple language like this. Zinc particles in the electrolyte are accelerated under the influence of electricity and begin to move from the anode to the cathode, i.e. to the body, and cover it with a thin but continuous layer. Galvanic galvanizing technology is rightfully considered the most effective way to resist corrosion, since the part is coated with a protective layer on all sides.
Causes of coating defects
There are several defects:
- Uneven shine - too much current or low solution temperature.
- Lack of shine - insufficient amount of sulfuric acid or anhydride.
- The appearance of brown spots is a lack of sulfuric acid or an excess of chromic anhydride.
- Low coating strength - low current or high electrolyte temperature.