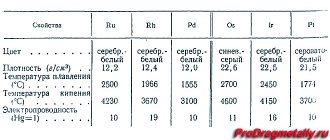
Chemical properties
- When heated, it easily loses water:
Sn(OH)2 →60−120oC SnO + H2O
- Shows amphoteric properties, dissolves in acids:
Sn(OH)2 + 3 HCl → H[SnCl3] + 2 H2O
- and alkalis:
Sn(OH)2 + NaOH → Na[Sn(OH)3]
| Tin compounds | |
| |
Bronze.
Long before they learned to extract tin in its pure form, an alloy of tin and copper was known - bronze, which was apparently obtained already in 2500-2000 BC.
Tin in ores is often found together with copper, so when copper was smelted in Britain, Bohemia, China and southern Spain, not pure copper was formed, but an alloy of it with a certain amount of tin. Early copper carpentry tools (chisel, adze, etc.) from Ireland contained up to 1% Sn. In Egypt, copper utensils of the 12th Dynasty (2000 BC) contained up to 2% Sn, apparently as an incidental impurity. The primitive practice of copper smelting was based on the use of a mixture of copper and tin ores, resulting in bronze containing up to 22% Sn. Table: Properties of b-TIN
| PROPERTIES of b-TIN | |
| Atomic number | 50 |
| Atomic mass | 118,710 |
| Isotopes | |
| stable | 112, 114–120, 122, 124 |
| unstable | 108–111, 113, 121, 123, 125–127 |
| Melting point, °C | 231,9 |
| Boiling point, °C | 2625 |
| Density, g/cm3 | 7,29 |
| Brinell hardness) | 3,9 |
| Content in the earth's crust, % (mass.) | 0,0004 |
| Oxidation states | +2, +4 |
Stannic acids
Tin dioxide hydrates are called stannous acids and are known in two modifications: as α-stannous acid and as β-stannic acid. α -Stannic acid
H2SnO3 can be prepared by reacting an aqueous ammonia solution with a solution of tin chloride SnCl4.
The formation of the white precipitate that falls is usually expressed by the equation
SnCl4+
4NH4OH = ↓ H2SnO3 + 4NH4Cl + H2O
As the precipitate dries, it gradually loses water until pure tin dioxide remains. Thus, no acid of a specific composition can be obtained. Therefore, the above formula for α-stannous acid is only the simplest possible. It would be more correct to depict the composition of this acid with the formula mSno2 • nH2O.
α-Stannic acid easily dissolves in alkalis, forming salts containing the complex anion [Sn(OH)6] - and called stannates:
H2SnO3 + 2NaOH + H2O = Na2[Sn(OH)6]
Sodium stannate is released from solution in the form of crystals, the composition of which can also be expressed by the formula Na2SnO3 • 3H2O. This salt is used as a mordant in dyeing and to weigh down silk. Silk fabrics treated with solutions of tin compounds before dyeing sometimes contain tin in amounts up to 50% by weight of the fabric.
Acids also dissolve α-stannous acid to form stannous salts. For example:
H2SnO3 + 4HCl ⇄ SnCl4 + 3H2O
When there is an excess of hydrochloric acid, SnCl4 adds two HCl molecules, forming complex chlorotin acid H2[SnCl6]. The ammonium salt of this acid, NH4[SnCl6], has the same uses as sodium stannate.
Tin
Tin, Sn, a metal, belongs to group IV of the periodic table (in the 7th row). Atomic weight Sn = 119.05 (O = 16). Specific gravity 7.293, at 13°. Melting point 232.7°, boiling point between 1450 and 1600°. In Europe it is found mainly in Cornwall. The richest tin deposits are located on a group of islands lying east of Sumatra, found in Saxony, Finland (Pitkaranta) and other places. The main ore is tin stone (cassiterite), SnO2. It is easily reduced, when heated with coal, into metallic tin. Tin represents a white metal, but somewhat duller and bluer than silver; at ordinary temperatures it is easily rolled into thin sheets (tin foil or stanniol). At low temperatures, white tin transforms into another allotropic modification - gray, specific gravity 5.8. At temperatures above 18°C, gray tin turns white. Without contact with gray tin, the transformation of white tin at ordinary temperatures occurs extremely slowly. The highest transition rate is observed at -47°. Therefore, in extreme cold, tin items (dishes, etc.) begin to crumble into powder. It can be assumed that at temperatures around 200° there is a third allotropic modification, since at 200° tin becomes so brittle that it can be pounded in a mortar. Tin does not oxidize at ordinary temperatures. It dissolves in hydrochloric acid, and tin chloride, SnCl2, is formed. When tin is exposed to nitric acid, a white powder of metatinic acid, H2SnO3, is obtained. When a hot solution of caustic potassium or soda acts on tin, tin-potassium or sodium salt is obtained: K2SnO3. Weak acids (acetic) have no effect on tin. The resistance of tin to oxygen is based on its use for tinning copper utensils and for coating iron (tinplate). Tin with oxygen forms tin oxide SnO and tin oxide, or tin anhydride, SnO2, respectively, which form other compounds, for example, SnS - a brown gray precipitate and SnS2 - a yellow precipitate, obtained by passing hydrogen sulfide into nitrous or tin oxide compounds SnS - also obtained by heating tin, an amalgam with sulfur and ammonia in the form of golden leaves, is called gold leaf or gold leaf and is used for bronzing various objects.
Among other compounds, we point out tin chloride, SnCl4, discovered in 1605 and named Spiritus fumans Libavii. It is obtained by burning tin in chlorine. The liquid boils at 113.9°, specific gravity 2.234. When exposed to water, it decomposes according to the equation SnCl4 + 4H2O = Sn(OH)4 + 4HCl. With potassium chloride, sodium and ammonium it forms salts of the composition SnCl4.2KСl, SnCl4.2NH4Cl. The last salt is called pinksaltz and is used in dyeing as a mordant. Stanniol, placed in a solution of pinksalt, quickly turns into gray tin. For tin chloride, there is its isomeric modification - metachlorine. For tin acids H2SnO3, two isomers are also known: tin and metatin acid. It can be assumed that metatinic acid (H2SnO3)x is a polymer of tin (H2SnO3).
I. Kb.
Extracting tin from ores is easier when the ore is in placer deposits than from a primary deposit. To purify the ore, it is manually sorted, then crushed, sifted and elutriated with water, producing concentrates rich in tin. Mechanical enrichment is very appropriate here, since the specific gravity of tin stone (6.8-7.0) significantly exceeds the specific gravity of most of its satellites. Further enrichment is achieved by firing, because the tin stone does not change, and the sulfur minerals are oxidized, turning into oxides; washing after firing can remove even more impurities; In addition, sulfur and arsenic (harmful impurities) are removed by burning. Roasting of ores sometimes precedes grinding if the ores are dense and hard; Then, after firing, they begin to wash the crushed mass. Sometimes the roasted ore is leached with water or dilute acid, which, dissolving various salts and oxides, do not act on the tin stone. By mechanical enrichment, roasting and subsequent washing, it is possible to obtain concentrates containing 50-70% tin from ores with 1-2% tin. Tin is separated in metallic form from enriched ore by reduction smelting in either shaft or flame furnaces. The reducing agent is carbon in the form of charcoal or in the form of lean coal with a low ash content (but not coke). The shaft furnace (when working on charcoal) is low, no more than 3 meters, so that a lot of iron is not recovered. Flux is never added to the smelted ore; if it contains tungsten and molybdenum, then add 2-4% lime. Air is blown into the shaft into two tuyeres, unheated. The fiery furnace, used in England for smelting tin, has an oval recessed hearth, filled with refractory clay, 1.4 m long and 1 m wide in the middle. 20-25 centners of concentrate (with 66-73% Sn) are added to the furnace at a time, mixed with ¼ - 1/5 parts (by weight) of anthracite or coal fines, with the addition of a small amount of limestone and fluorspar to form slag. Melt with the doors closed, gradually raising the temperature and stirring the mixture from time to time. The liquid slag formed is drained several times, sprinkling coal powder over the melting mass. Recovery ends in 6-8 hours. A nest (recess) is made next to the hearth, into which the smelted tin is released.
Tin smelted directly from ore is not pure; it contains some iron, copper, arsenic, antimony, tungsten. Purification of impurities or refining of tin is carried out in factories in 2 ways; German and English. The German method is zeigering; in it, the separation is based on the difference in fusibility of tin and mixed metals. In the English method, in addition to zengering, teasing is carried out, as in the refining of copper (Cf. XXIX, 4921).
Chemically pure tin is obtained from commercial tin as follows: tin is dissolved in hydrochloric acid; in this case, copper, antimony, lead and part of the tin remain in the sediment; Some of the arsenic is released in the form of arsenic hydrogen. The solution produces tin and zinc. The boiling solution is precipitated with soda; the precipitate is treated with nitric acid: then the tin turns into insoluble metatinic acid, and the zinc dissolves. Tinic acid is thoroughly washed and reduced with black flux, i.e., a mixture of coal and potash.
To extract tin from tinplate waste, they now often resort to electrolysis, performed in both an acidic and an alkaline bath: large factories prefer electrolysis of an alkaline solution (in the presence of caustic soda). tin dissolves from the tin serving as the anode and is deposited on the cathode, if you do not stop at obtaining tin preparations.
The main use of tin is in the preparation of various alloys, for example, bronze (see), white metals. A lot of tin is used in soldering (see) and tinning (see), as well as for coating iron (tinplate) and wrapping. Part of the tin is converted into chemical preparations: tin salt (tin chloride), oxidized salt (SnCl4.5H2O), tin-sodium salt, mussed gold (SnS2), etc.
Tin production in tons.
| 1900 | 1905 | 1907 | 1908 | 1909 | |
| Delivery by sea | 48630 | 59500 | 56550 | 63690 | 61540 |
| England from local ores | 4336 | 4538 | 44780 | 5127 | 5000 |
| England from foreign ores | 3575 | 8500 | 10020 | 11614 | 11890 |
| Buying on Bank Island in Holland | 12000 | 10200 | 11440 | 11710 | 12150 |
| Germany | 2031 | 5233 | 5838 | 6375 | 8990 |
| Australia | 3800 | 5800 | 7100 | 6700 | 6450 |
| Billiton shopping in Holland and Java | 5913 | 2760 | 2260 | 2270 | 2280 |
| Total production | 80300 | 96600 | 97700 | 107500 | 108300 |
| Cost in thousands of marks | 215000 | 227000 | 339000 | 292000 | 298000 |
| Cost of 1 pood in marks | 4308 | 3805 | 56,8 | 41,1 | 45,1 |
German tin mining is carried out mainly from Bolivian ores. In Germany, up to 75,000 tons of tinplate waste and about 15,000 tons of tin (corresponding tin preparations) are processed annually, i.e., approximately 70% of the total German tin consumption. In the rest of Europe, about 25,000 thousand tin waste is converted: in the United States about 60,000 tons, so in round numbers - up to 160,000 tons of tinplate with 3,000 - 5,500 tons of tin. Most of the tin metal is converted into tin preparations, so that German production of metal tin is reduced to only 6400-9000 tons.
From 350 to 430 thousand poods of tin are imported into Russia annually at a cost of 110,000 rubles. The cost of tin varies per pood from 26 to 34 rubles. at normal times.
E. Orlov.
Preparation of organotin compounds
Organotin compounds can be synthesized using numerous methods. The classic reaction is the Grignard reagent with tin halides, for example, with stannous tetrachloride. An example is the synthesis of tetraethyltin:
$4 EtMgBr + SnCl_4 \to Et_4Sn + 4 MgClBr$
Symmetrical organotin compounds can then be converted to various mixed chlorides by redistribution reactions (also known as the "Kocheshkov reaction"):
$3 R_4Sn + SnCl_4 \to 4 R_3SnCl$
$R_4Sn + SnCl_4 \to 2 R_2SnCl_2$
$R_4Sn + 3 SnCl_4 > 4 RSnCl_3$
A related method involves the redistribution of tin halide with organoaluminum compounds.
Mixed organohalide tin compounds can be converted into mixed organic derivatives, as shown by reaction with dibutyllithium:
$Bu_2SnCl_2 + 2 C_2H_3MgBr \to Bu_2Sn(C_2H_3)_2 + 2 MgBrCl$
Organotin hydrides are prepared by reduction of mixed alkyl chlorides. For example, the reaction of dibutyltin dichloride with lithium aluminum hydride gives dibutyltin dihydride:
Figure 6.
Similar combinations of sodium alkyl compounds with tin halides produce tetraorganotin compounds.
Alloys.
One third of the tin is used to make solders. Solders are alloys of tin mainly with lead in different proportions depending on the purpose. An alloy containing 62% Sn and 38% Pb is called eutectic and has the lowest melting point among the alloys of the Sn – Pb system. It is included in compositions used in electronics and electrical engineering. Other lead-tin alloys, such as 30% Sn + 70% Pb, which have a wide solidification range, are used for pipeline soldering and as filler material. Lead-free tin solders are also used. Alloys of tin with antimony and copper are used as antifriction alloys (babbitt, bronze) in bearing technology for various mechanisms. Modern tin-lead alloys contain 90–97% Sn and small additions of copper and antimony to increase hardness and strength. Unlike early and medieval lead-containing alloys, modern cookware made from tin alloys is safe to use.
Determination of the structure of the valence electron layer of elemental atoms
Problem 188. The structure of the valence electron layer of an element’s atom is expressed by the electronic formula: a) 5s25p4; b) 3d54s1. Determine the serial number and name of the element.
Solution: a) The valence electron layer 5s25p4 indicates that the atom of the element has five electronic energy levels, which means the atom is located in the fifth period.
The presence of two 5s and four 5p electrons on the outer energy level indicates that this element belongs to the family of p-elements and is located in the sixth group of the main subgroup of D. I. Mendeleev’s periodic system. In the fifth period of the sixth group there is an element with serial number 52 (tellurium).
b) The electronic configuration of the valence layer 3d54s1 indicates that the atom is in the fourth period (n = 4), belongs to d-elements (presence of a 3d sublevel) and is an element of the sixth group of the secondary subgroup. This state corresponds to an element with serial number 24 (chrome).
Answer: Te; Cr.
Task. 189. The electronic structure of an atom is described by the formula: 1s22s22p63s23p63d64s2.
What element is this? Solution: Since the number of electrons in an atom of an element is equal to its serial number in the table D.I.
Mendeleev, then for an element with an electronic structure described by the formula 1s22s22p63s23p63d64s2, the atomic number is 26 (the total number of electrons is 26). At number 26 in D.I. Mendeleev’s table is iron.
Problem 190. Write the electronic formulas of the ions: a) Sn2+; b) Sn4+; c) Mn2+; d) Cu2+; e) Cr3+; e) S2-. Solution: a) The electronic formula of tin is: 1s22s22p63s23p63d104s24p64d105s25p2. Having donated two atoms from the 5p sublevel, tin turns into the Sn2+ ion, which has the electronic formula:
1s22s22p63s23p63d104s24p64d105s25p0
b) The tin atom, having given up four electrons, two from the 5p sublevel and two from the 4s sublevel, the tin atom turns into the Sn4+ ion. The electronic formula of the tin ion Sn4+ is: 1s22s22p63s23p63d104s24p64d105s05p0.
c) The electronic formula of manganese is: 1s22s22p63s23p63d54s2. When two electrons are lost from the 4s sublevel, the manganese atom turns into an Mn2+ ion with the electronic formula: 1s22s22p63s23p63d54s0
d) The copper atom has the electronic formula: 1s22s22p63s23p63d104s1. by donating one electron from the 4s sublevel and one from the 3d sublevel, the copper atom turns into a Cu2+ ion, the electronic formula of which will be: 1s22s22p63s23p63d9.
e) The chromium atom has the following electronic formula: 1s22s22p63s23p63d54s1. By donating one electron from the 4s sublevel and two from the 3d sublevel, the chromium atom turns into a Cr3+ ion, the electronic formula of which will be: 1s22s22p63s23p63d34s0.
f) The electronic formula of the sulfur atom is: 1s22s22p63s23p4. By adding two missing electrons to the 3p sublevel, the sulfur atom turns into an S2- ion, the electronic formula of which will be: 1s22s22p63s23p6.
Problem 191. Which elements of which periods have the electrons of the outer layer characterized by the value n + l = 5?
Solution:
The value of quantum numbers n + l = 5 means that the electrons of the outer layer of elements can be at the fifth energy level and s-sublevel (5 + 0 = 5) or at the fourth energy level and p-sublevel (4 +1 = 5).
Thus, for elements of periods IV and V, the electrons of the outer layer are characterized by the value n + l = 5.
Problem 192. List electronic analogues among the elements of group VI of the periodic table of elements.
Production
During the production process, the ore-bearing rock (cassiterite) is crushed to particle sizes of an average of ~ 10 mm in industrial mills, after which cassiterite, due to its relatively high density and mass, is separated from the waste rock by vibration-gravity method on dressing tables. In addition, the flotation method of ore enrichment/purification is used. In this way, it is possible to increase the tin content in the ore to 40-70%. Next, the concentrate is roasted in oxygen to remove impurities of sulfur and arsenic. The resulting tin ore concentrate is smelted in furnaces. During the smelting process, it is restored to a free state through the use of charcoal in the reduction, the layers of which are laid alternately with layers of ore, or aluminum (zinc) in electric furnaces: SnO2 + C = Sn + CO2. Particularly pure tin of semiconductor purity is prepared by electrochemical refining or the zone melting method.
Tin(II) chloride, or stannous chloride
SnCl2•2H2O is obtained by dissolving tin in hydrochloric acid and forms colorless crystals with two molecules of water of crystallization. When heating or strongly diluting a SnCl2 solution with water, partial hydrolysis occurs with the formation of a precipitate of the main salt:
SnCl2 + H2O ⇄ ↓SnOHCl + HCl
Stannous chloride is an energetic reducing agent.
So, for example, ferric chloride FeCl3 is reduced by it into ferric chloride FeCl2:
2FeCl3+ SnCl2 = 2FeCl2 + SnCl4
When tin chloride acts on a solution of sublimate, a white precipitate of calomel is formed. With an excess of SnCl2, the reduction goes even further and metallic mercury is obtained:
2HgCl2 + SnCl2 = ↓ Hg2Cl2 + SnCl4
Hg2Cl2 + SnCl2 = 2Hg + SnCl4
Tetravalent tin compounds. Tin dioxide
SnO2 occurs naturally in the form of tinstone, the most important ore of tin. It can be produced artificially by burning the metal in air or by oxidizing it with nitric acid, followed by calcination of the resulting product. Used for preparing various white glazes and enamels.
Electronic structure of the atom
Electronic formula
1S22S22P63S23P63D104S24P64D104F05S25P2
Sn - tin. Serial number 50, period 5, group IV, main (A) subgroup.
The serial number of tin is 50, and the relative atomic mass Ar = 119 (rounded value). Accordingly, the charge of the nucleus of its atom is +50 (number of protons). Therefore, the number of neutrons in the nucleus is N=Ar-Z=69. Since the atom is electrically neutral, the number of electrons contained in a tin atom is also 50.
The valence sublevels in the electronic formula of this chemical element are 5S and 5P: 5S25P2. Tin belongs to the P-elements, because this element is the last to fill the fifth electronic layer, the 5P sublevel.
- Possibility of “electron failure effect”:
- 50Sn
- 5S 4D
- Since the 4D sublevel is completely filled with electrons, the “electron failure effect” is not observed
- Sets of quantum numbers for all valence electrons:
- S1:n=5, l=0, ml=0, ms=+1/2;
- S2:n=5, l=0, ml=0, ms=-1/2; -1 0 1
- P2:n=5, l=1,ml=0,ms=+1/2.
- P1:n=5, l=1, ml=-1, ms=+1/2; +50Sn
Tin is a metal because its atoms give up electrons, becoming positive ions. Since tin is located near the boron-astatine diagonal, it has dual properties: in some compounds it behaves like a metal, in others it behaves like a non-metal (amphoteric oxides and hydroxides).
Since tin atoms contain 4 electrons on the outer layer, they can donate them, thereby acquiring an oxidation state of +4 (showing reducing properties). Tin can also take on the +2 oxidation state.
Sn>Sn*
5S 5P 5S 5P
co=+2 co=+4
- According to Hund's rule, the total spin number s must be maximum. Let's place 2 electrons in the P-atomic orbital
- 1) 2)
- = +1/2 — 1/2 = 0 = +1/2 +1/2 = 1
- Since in the second option s=max, then two electrons are located in the P-atomic orbital in the same position as in the second option.
According to the principle of least energy, electrons in the ground state fill orbitals in order of increasing energy levels of the orbitals.
In the same level, the energy of sublevels increases: EsSn*
- 5S 5P 5S 5P
- K=2 K=*4 SP3q4 hybridization
- The tin atom in the excited state has a covalency of 4.
With hydrogen, tin forms the hydride SnH4; with halogens, compounds such as SnX2 and SnX4.
Compounds of the SnX2 type are due to the presence of a lone pair of electrons in tin. Compounds like SnX4 and SnH4 have SP3q4 hybridization and have a tetrahedron shape.
Source: https://him.bobrodobro.ru/10833
1.Chemical element tin(Sn)
1.1.
Electronic formula of this chemical element:
1S22S22P63S23P63D104S24P64D104F05S25P2
Abbreviated electronic formula: 4D105S25P2
In the 5th period, electrons fill first the 5S sublevel, then the 4D sublevel, then the 5P sublevel. Starting from the 3rd period, there is a discrepancy between the number of electrons at the energy level and the number of electrons in a given period, which can be explained by the principle of least energy.
In accordance with this principle, when energy levels are filled, a delay effect is observed. Electrons in this state fill the orbitals in order of increasing energy level of the orbitals.
In accordance with Klechkovsky's rule, the increase in energy and, accordingly, the filling of orbitals occurs in increasing order of the sum of quantum numbers (n+l), and in the case of an equal sum (n+l) in increasing order of the number n.
4D(4+2)=6 5S(5+0)=5 5P(5+1)=6
The 4D and 5P sublevels have the same values (n+l), but the 4D sublevel is energetically more favorable, since it has a lower n value. Therefore, these sublevels are filled in in the following order: 5S, 4D, 5P. The 5th period is filled in similarly to the 4th.
1.2.
I. Sn – tin. Serial number 50, period 5, group IV, main (A) subgroup.
- The serial number of tin is 50, and the relative atomic mass Ar = 119 (rounded value). Accordingly, the charge of the nucleus of its atom is +50 (number of protons). Therefore, the number of neutrons in the nucleus is N=Ar-Z=69. Since the atom is electrically neutral, the number of electrons contained in a tin atom is also 50.
- The element tin is in the 5th period of D.I. Mendeleev’s periodic table, which means that all the electrons of the atom are located at five energy levels. Also, the number of electrons that are in a given period is determined by the period number. Their number is equal to: Xe=2n2=2*52=50.
- The group number (IV) indicates that the maximum oxidation state of the metal is +4.
- Tin belongs to group IV of the main (A) subgroup, therefore, tin is a P-element.
Reactions of organotin compounds
The important reactions discussed above tend to focus on organotin halides and pseudohalides with nucleophiles. In the field of organic synthesis, the Stille reaction is considered extremely important. It consists of a coupling reaction with $sp2$-hybridized organic halides catalyzed by palladium:
Figure 7.
Note 2
Organotin compounds are also widely used in radical chemistry (eg, radical cyclization, Barton-McCombie deoxygenation, Barton decarboxylation, etc.).