4.1
Average rating: 4.1
Total ratings received: 284.
4.1
Average rating: 4.1
Total ratings received: 284.
In solid crystalline bodies, molecules are arranged in an orderly manner, forming a crystal lattice, the structure of which is reproduced throughout the entire volume - this arrangement of particles is called long-range order. When a body is heated, the kinetic energy of the molecules increases, and when the melting temperature is reached, the lattice structure begins to collapse, the solid body loses its shape - the melting process begins. When cooled, solidification occurs - a transition from the liquid phase to the solid phase.
Why does melting happen?
In the solid state, molecules and atoms are located at lattice sites, performing continuous vibrations near a fixed position. Such vibrations do not disturb the crystal structure. The strength of the lattice is provided by intermolecular bonds. In the process of heating a body, thermal energy is transferred, which is converted into the internal energy of molecules, increasing their speed and vibration frequency. When a certain critical value of temperature Tmelt (melting temperature) is reached, intermolecular bonds are broken, molecules leave their places, which leads to a change in the shape of the body, which begins to transform into a liquid state.
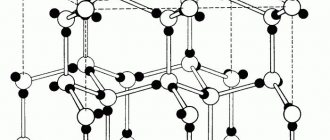
Rice. 1. Examples of the structure of crystal lattices: graphite, diamond, NaCl.
So, melting is the process of transition from a solid to a liquid state.
Transitions between phases
When studying the phenomena of melting and crystallization, you should become familiar with the concept of the phase of a substance. It is defined as a state of matter that is determined by its specific structure and physical and chemical properties. It is important not to confuse this concept with the state of aggregation. For example, the pure element iron can be in one state of aggregation, but have different phases.
So, being solid, it can be in the following forms:
- alpha low temperature - has a body-centered cubic lattice and has magnetic properties;
- gamma - has a face-centered cubic crystal lattice, non-magnetic;
- delta high temperature - has the same lattice type as alpha iron, but is non-magnetic.
Transformations of the first kind
In physics, many types of transformations between phases have been studied, which are accompanied by a certain thermal effect. Such transitions are called transformations of the first kind. The following are distinguished:
- from solid to liquid - melting, vice versa - crystallization;
- from solid to gaseous - sublimation, vice versa - desublimation;
- from liquid to gaseous - boiling, vice versa - condensation.
Plasma, which also refers to one of the states of aggregation, is not shown here. It is characterized by ionization processes.
In addition to transitions of the first kind, there are transformations of the second kind. The latter are not characterized by a thermal effect, but are associated only with a change in some properties of the substance, for example, magnetism or electrical conductivity.
Gibbs energy
To understand the graphs of transformations of a substance between different phases, it is necessary to master the concept of Gibbs energy. This physical quantity characterizes the stability of the phase under the conditions under consideration. By the latter, in most cases, they mean temperature and pressure. To change the Gibbs energy ΔG, you can write the following thermodynamic expression: ΔG = ΔH - T * ΔS.
Here ΔH is the change in enthalpy or latent heat during the phase transformation being studied, ΔS is the change in entropy or measure of disorder during the physical process, T is the absolute temperature value.
It should be remembered that any thermodynamic process is beneficial from an energy point of view if, as a result, the change in the Gibbs free energy turns out to be negative (ΔG<0), that is, the value of G decreases.
In addition to the energy factor of the randomness of transformation, there is also a kinetic factor. Any substance, no matter what its state of aggregation, is characterized by a certain microscopic structure. During the phase transformation process, this structure changes. To do this, it is necessary that the particles participating in it have a certain reserve of kinetic energy, which will allow them to perform spatial rearrangement. Understanding the kinetic factor plays a key role in amorphous and metastable transformations.
What is hardening
Observations show that if the molten substance is cooled, then when the temperature Tts (solidification temperature) is reached, the reverse process of transition from the liquid to the solid state begins. This phase transition is called solidification or crystallization. It has been experimentally proven that for crystalline solids Tmelt = Tres. “Hot” molecules lose speed when cooled and give off heat to the environment. The internal energy decreases, and particles, under the influence of molecular interaction forces, begin to “occupy” permanent positions, restoring the lattice structure.
The processes of melting and solidification do not occur abruptly, but gradually, so that solid and liquid components can coexist simultaneously. Experiments show that until the melting (or solidification) of the entire mass of a substance is completed, its temperature remains constant.
Metals whose melting point is greater than 16500C are called refractory. For example, the melting point of tungsten is 33700C. Therefore, long-lasting filaments for lamps are made from it. Refractory metals and their alloys are indispensable in rocket science, nuclear energy, metallurgy, space technology - wherever high heat-resistant properties are required.
Solids and liquids
Every person encounters these aggregate states of matter every day. In order to clearly understand what processes occur during the melting of solids, crystallization of liquids and their heating, it is necessary to know about the features of the internal structure of these states of aggregation at the atomic-molecular level. Liquids and solids have one thing in common - they are incompressible. Their other characteristics differ significantly.
Crystalline and amorphous state
Despite the great variety of solids, their internal structure belongs to one of two groups . The following are distinguished:
- crystals;
- amorphous.
Both are characterized by a certain physical form and the ability to resist external mechanical loads.
Crystals consist of ordered cells of atoms and molecules in space so that a regular geometric lattice is formed, which is called a crystal lattice. Using translations to specific vectors, you can get from one lattice node to any other. The strict periodic structure determines the anisotropy of the physical properties of crystals. Examples of these are table salt, steel, ice and more.
Pure crystals are difficult to find in nature , so they always contain impurities, vacancies, dislocations, that is, defects that disrupt their ideal geometric structure.
Amorphs are substances in which the structural order is disrupted. They do not have long-range order, like crystals. However, they are characterized by short-range order, which is determined by the chemical properties of the corresponding elements. Amorphs are isotropic. An example is glass.
Fluid incompressible substances
We are talking about liquids. Their typical structure is a chaotic arrangement of particles that are capable of jumping from one position to another in short periods of time. This fact distinguishes them from solid bodies, where each particle has a very specific place around which it oscillates.
The atoms and molecules of a liquid are weakly bonded to each other, unlike crystals and amorphs. This allows the substance to easily take an arbitrary shape under any non-volumetric mechanical influences on it. On the other hand, particles in liquids are located at a sufficiently close distance from each other to interact using van der Waals bonds and prevent them from scattering in all directions, like gas molecules.
If a liquid is cooled sharply, then all its particles can be frozen in their positions. The result is an amorphous solid state. It is metastable from an energy point of view, however, to transition to a stable state it is necessary to expend a certain activation energy (kinetic factor).
Graphical representation of melting and solidification processes
A graph of the melting and solidification of crystalline solids provides a visual representation of the time dependence of these phase transitions.
Rice. 2. Water-ice melting and solidification graph.
Ordinary water is a good example to illustrate the phenomena discussed. In the presented graph, time t is plotted along the abscissa axis, and temperature is plotted along the ordinate axis. Let initially, at the moment of time t = 0, when the temperature of the ice (crystal) was equal to -400C, the heat supply - heating - will begin. Let us next consider the time dependence of the temperature dependence T(t):
- In section AB, from -400C to 00C (melting temperature of ice), ice exists in crystalline form;
- Section BC - the melting stage occurs, both ice and water are present. The temperature remains constant, equal to 00C;
- CD - at point C melting has ended, there is only a liquid phase - water;
- DE - at point D heating has stopped, cooling occurs up to point E, i.e. up to a temperature of 00C. Only liquid water is present;
- EF - at point E, solidification begins, ice crystals appear, but at the same time there is also a liquid phase. The temperature remains constant, equal to 00C;
- FK - at point F complete solidification has occurred, only ice remains in crystalline form, the temperature of which gradually decreases.
Properties of water
Before talking about the melting temperature of ice, it is worth considering the basic properties of this unique liquid.
So, water has the following properties:
- Lack of color.
- No smell.
- Lack of taste (however, high-quality drinking water has a pleasant taste).
- Transparency.
- Fluidity.
- The ability to dissolve various substances (for example, salts, alkalis, etc.).
- Water does not have its own permanent shape and is able to take the shape of the vessel into which it falls.
- Ability to be purified by filtration.
- When heated, water expands and when cooled, it contracts.
- Water can evaporate into steam and freeze to form crystalline ice.
This list shows the main properties of water. Now let's figure out what the features of the solid state of aggregation of this substance are, and at what temperature ice melts.
What is specific heat of fusion
The specific heat of fusion λ (Greek letter “lambda”) is a physical quantity equal to the amount of heat that must be transferred to a solid body weighing 1 kg in order to completely transform it into the liquid phase. The formula for the specific heat of fusion looks like this:
$ λ ={Q \over m}$ (1)
Where:
m is the mass of the melting substance, kg;
Q is the amount of heat transferred to the substance during melting, J.
The values of λ for different substances are determined experimentally. The dimension λ follows from formula (1):
$ [λ] = { [J]\over [kg] } $ (2)
Knowing λ, we can calculate the amount of heat Q that must be imparted to a body of mass m for its complete melting:
$Q={ λ * m}$ (3)
When cured, exactly the same amount of heat will be returned to the environment.
When heated, some substances bypass the melting stage and immediately evaporate. This process is called sublimation or sublimation. An example of such a substance is crystalline iodine. The reverse transition from the gaseous state, which occurs without the formation of a liquid phase, is called desublimation. Examples of such transitions are the formation of iodine crystals from iodine vapor and the precipitation of frost and snowflakes from water vapor in the air.
Rice. 3. Formation of frost patterns on glass.
Water on Earth
This is not to say that water vapor and ice are rarely encountered in everyday life. However, the most common is the liquid state - ordinary water. Experts have found that there is more than 1 billion cubic kilometers of water on our planet. However, no more than 3 million km3 of water belongs to fresh water bodies. A fairly large amount of fresh water “rests” in glaciers (about 30 million cubic kilometers). However, melting the ice of such huge blocks is far from easy. The rest of the water is salty, belonging to the seas of the World Ocean.
Water surrounds modern man everywhere, during most daily procedures. Many believe that water supplies are inexhaustible, and humanity will always be able to use the resources of the Earth's hydrosphere. However, this is not the case. The water resources of our planet are gradually depleted, and within a few hundred years there may be no fresh water left on Earth at all. Therefore, absolutely every person needs to treat fresh water with care and save it. After all, even in our time there are states in which water reserves are catastrophically small.
Melting of amorphous bodies
Amorphous bodies do not have a specific melting point. The structure of amorphous bodies is more like a very viscous liquid than a crystalline solid. When heated, they will become more fluid, increasingly exhibiting the property of a liquid. At the same time, the fragility inherent in the solid state will disappear. Simultaneously with melting, the temperature of amorphous bodies will increase.
Important! Simultaneously with melting, the temperature of amorphous bodies will continuously increase. Because such bodies do not have a specific melting point.
Examples of amorphous bodies
- rosin (coniferous tree resin);
- glass;
- ebonite;
- sealing wax;
- various plastics;
Note: Ebonite (“Ebenos” in ancient Greek - ebony) is vulcanized rubber with the addition of a large amount of sulfur, up to 50% of the mass of the rubber. The color of ebonite is usually dark brown or black. This material does not conduct electrical current - that is, it is a good insulator.
Quantity of heat
In a science such as physics, the concept of quantity of heat is often used. This value shows the amount of energy required to heat, melt, crystallize, boil, evaporate or condense various substances. Moreover, each of the listed processes has its own characteristics. Let's talk about how much heat is required to heat ice under normal conditions.
To heat ice, you must first melt it. This requires the amount of heat needed to melt the solid. The heat is equal to the product of the mass of ice and the specific heat of its melting (330-345 thousand Joules/kg) and is expressed in Joules. Let's say that we are given 2 kg of hard ice. Thus, to melt it, we need: 2 kg * 340 kJ/kg = 680 kJ.
After this, we need to heat the resulting water. The amount of heat for this process will be a little more difficult to calculate. To do this, you need to know the initial and final temperatures of the heated water.
So, let's say that we need to heat the water resulting from melting ice by 50 °C. That is, the difference between the initial and final temperatures = 50 °C (initial water temperature – 0 °C). Then you should multiply the temperature difference by the mass of water and its specific heat capacity, which is equal to 4,200 J*kg/°C. That is, the amount of heat required to heat water = 2 kg * 50 °C * 4,200 J*kg/°C = 420 kJ.
Then we find that to melt the ice and subsequently heat the resulting water we will need: 680,000 J + 420,000 J = 1,100,000 Joules, or 1.1 Megajoule.
Knowing at what temperature ice melts, you can solve many difficult problems in physics or chemistry.
Snow and ice
Ice is a solid crystalline substance that has a rather unstable structure. It, like water, is transparent, colorless and odorless. Ice also has properties such as fragility and slipperiness; it is cold to the touch.
Snow is also frozen water, but it has a loose structure and is white in color. It is snow that falls every year in most countries of the world.
Both snow and ice are extremely unstable substances. It doesn't take much effort to melt the ice. When does it start to melt?
Melting of crystalline bodies
In order for a crystalline body to begin to melt, it must be heated to a certain temperature. Some crystalline bodies will melt at low temperatures, while others will melt at high temperatures. That is, each substance has its own melting point. It can be found in the physics reference book. At the same time, until the substance melts, its temperature will not change.
Important! Crystalline solids have a specific melting point. Until the crystalline substance is completely melted, its temperature will not change!
Notes:
- Crystalline substances melt at the same temperature at which they will turn into a solid (crystallize).
- In order for a liquid substance to begin to crystallize, it must first cool to a certain temperature.
- The melting point and the crystallization temperature are the same temperature.
Examples of crystalline solids
- ice;
- lead;
- aluminum;
- mercury;
- iron;
- gold;
- silver;