Martensite and martensitic transformation in steels
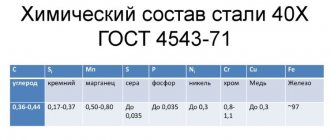
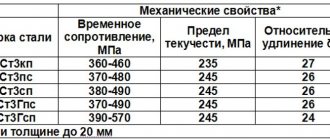
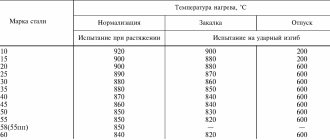
Martensite is a supersaturated solid solution of carbon in α-iron (α-Fe). Read what austenite, cementite, ferrite and pearlite are here. When eutectoid steel (0.8% carbon) is heated above point A1, the original pearlite structure will transform into austenite. In this case, all the carbon present in the steel will dissolve in austenite, i.e. 0.8%. Rapid cooling at a supercritical rate (see figure below), for example in water (600 °C/sec), prevents the diffusion of carbon from austenite, but the fcc crystal lattice of austenite will rearrange into the tetragonal lattice of martensite. This process is called martensitic transformation. It is characterized by the shear nature of the restructuring of the crystal lattice at a cooling rate at which diffusion processes become impossible. The product of martensitic transformation is martensite with a distorted tetragonal lattice. The degree of tetragonality depends on the carbon content in the steel: the more it is, the greater the degree of tetragonality. Martensite is a hard and brittle structure of steel. Found in the form of plates, under a microscope they look like needles.
The hardening temperature for most steels is determined by the position of the critical points A1 and A3. In practice, the hardening temperature of steels is determined using steel graders. How to choose the hardening temperature of steel, taking into account points Ac1 and Ac3, read the link.
Microstructure of steel after hardening
Most steels after hardening are characterized by the structure of martensite and retained austenite, the amount of the latter depending on the carbon content and the qualitative and quantitative content of alloying elements. For structural steels of medium alloying, the amount of retained austenite can be in the range of 3-5%. In tool steels this amount can reach 20-30%.
In general, the structure of steel after hardening is determined by the final requirements for the mechanical properties of the product. Along with martensite, after quenching, ferrite or cementite may be present in the structure (in case of incomplete quenching). When steel is isothermally hardened, its structure may consist of bainite. The structure, final properties and hardening methods of steel are discussed below.
Partial hardening of steel
Partial quenching is called quenching, in which the cooling rate is not sufficient for the formation of martensite and it turns out to be below critical. This cooling rate is indicated by the blue line in the figure. During partial hardening, the “nose” of the C-curve steel seems to be touched. In this case, in the structure of the steel, along with martensite, troostite will be present in the form of black island inclusions.
The microstructure of partially hardened steel looks something like this:
Partial hardening is a defect that is eliminated by complete recrystallization of the steel, for example, during normalization or during reheating for hardening.
Steel hardening
Hardening is a heat treatment operation of metal. It consists of heating the metal to a critical temperature at which the crystal lattice of the material changes , or to a temperature at which the phase dissolves in the matrix, which exists at a low temperature.
It is important to understand:
- After reaching a critical temperature, the metal undergoes rapid cooling.
- After hardening, the steel acquires the structure of martensite (named after Adolph Martens) and therefore becomes hard.
- Hardening increases the strength of steel. The metal becomes even harder and more wear-resistant.
- A distinction should be made between conventional quenching of the material and quenching to obtain excess vacancies.
Hardening modes differ in the speed of the process and heating temperature. There are also differences in the duration of exposure at a given temperature and cooling rate.
Incomplete hardening of steels
Quenching at temperatures lying between A1 and A3 (incomplete quenching) retains in the structure of hypoeutectoid steels, along with martensite, part of the ferrite, which reduces the hardness in the quenched state and worsens the mechanical properties after tempering. This is understandable, since the hardness of ferrite is 80HRC, and the hardness of martensite depends on the carbon content and can be more than 60HRC. Therefore, these steels are usually heated to temperatures 30–50 °C above A3 (full hardening). In theory, incomplete hardening of steels is not permissible and is considered a defect. In practice, in some cases, incomplete quenching can be used to avoid quenching cracks. Very often this concerns hardening with high frequency currents. With such hardening, it is necessary to take into account its feasibility: type of production, annual program, type of product responsibility, economic justification. For hypereutectoid steels, quenching at temperatures above A1 but below Acm produces excess cementite in the structure, which increases the hardness and wear resistance of the steel. Heating above the temperature Acm leads to a decrease in hardness due to the dissolution of excess cementite and an increase in retained austenite. In this case, the austenite grain grows, which also negatively affects the mechanical characteristics of the steel.
Thus, the optimal quenching for hypoeutectoid steels is quenching from a temperature 30–50 °C above A3, and for hypereutectoid steels – at 30–50 °C above A1.
The cooling rate also affects the hardening result. The optimal cooling medium is one that quickly cools the part in the temperature range of minimum stability of supercooled austenite (in the range of the nose of the c-curve) and slowly in the temperature range of martensitic transformation.
Cooling stages during hardening
The most common quenching media are water of various temperatures, polymer solutions, alcohol solutions, oil, molten salts. When hardening in these environments, several cooling stages are distinguished:
— film cooling, when a “steam jacket” is formed on the surface of the steel;
- nucleate boiling, which occurs with the complete destruction of this steam jacket;
— convective heat transfer.
More details about the cooling stages during quenching can be found in the article “Characteristics of quenching oils”
In addition to liquid quenching media, cooling in a gas flow of different pressures is used. It can be nitrogen (N2), helium (He) and even air. Such quenching media are often used in vacuum heat treatment. Here it is necessary to take into account the fact of the possibility of obtaining a martensitic structure - the hardenability of steel in a certain environment, i.e. the chemical composition of the steel on which the position of the c-curve depends.
Cooling methods during hardening
When steel products are rapidly cooled during hardening, there is a risk of large internal stresses occurring, which leads to warping of the material and sometimes cracks. To avoid this, where possible, it is better to cool steel parts in oil. Carbon steel, for which such cooling is not possible, is better cooled in water.
In addition to the cooling environment, the internal stress of steel products is affected by how they are immersed in the cooling environment. Namely:
- products with a thick and thin part in the quenching liquid with the bulky part first;
- If the product has an elongated shape (drills, taps), it must be immersed strictly vertically, otherwise they may warp.
Sometimes it is not necessary to harden the entire part, but only part of it. Then local hardening is applied. The product is not completely heated, but the entire part is immersed in the quenching liquid.
Factors influencing the position of c-curves:
- Carbon. Increasing the carbon content to 0.8% increases the stability of supercooled austenite, and accordingly the c-curve shifts to the right. When the carbon content increases above 0.8%, the c-curve shifts to the left;
— Alloying elements. All alloying elements increase the stability of austenite to varying degrees. This does not apply to cobalt; it reduces the stability of supercooled austenite;
— Grain size and homogeneity. The larger the grain and the more uniform its structure, the higher the stability of austenite;
— An increase in the degree of distortion of the crystal lattice reduces the stability of supercooled austenite.
Temperature affects the position of c-curves through all of the above factors.
Methods of hardening steels
In practice, various cooling methods are used depending on the size of the parts, their chemical composition and the required structure (diagram below).
Diagram: Cooling rates for different methods of hardening steels
Continuous hardening of steel
Continuous hardening (1) is a method of cooling parts in one environment. After heating, the part is placed in a quenching medium and left there until completely cooled. This technology is the most common and is widely used in mass production. Suitable for almost all types of structural steels.
Hardening in two environments
Quenching in two environments (speed 2 in the figure) is carried out in different quenching environments, with different temperatures. First, the part is cooled in the temperature range, for example, 890–400 °C, for example in water, and then transferred to another cooling medium - oil. In this case, the martensitic transformation will already occur in an oil environment, which will lead to a decrease in the leash and warping of steel. This hardening method is used for heat treatment of stamping tools. In practice, the opposite technological technique is often used - first the parts are cooled in oil and then in water. In this case, the martensitic transformation occurs in oil, and the parts are moved into water for faster cooling. This saves time on implementing the hardening technology.
Step hardening
During stepwise quenching (speed 3), the product is cooled in a quenching medium having a temperature higher than the martensitic transformation temperature. In this way, a certain isothermal holding is obtained before the transformation of austenite into martensite begins. This ensures uniform temperature distribution over the entire cross-section of the part. This is followed by final cooling, during which the martensitic transformation occurs. This method produces hardening with minimal internal stress. Isothermal holding can be done just below the temperature Mn, after the start of the martensitic transformation (speed 6). This method is more difficult from a technological point of view.
Isothermal hardening of steels
Isothermal hardening (speed 4) is done to obtain the bainitic structure of the steel. This structure is characterized by an excellent combination of strength and plastic properties. During isothermal hardening, parts are cooled in a bath of molten salts, which have a temperature 50–150 °C above the martensite point Mn, maintained at this temperature until the end of the transformation of austenite into bainite, and then cooled in air.
When hardening onto bainite, it is possible to obtain two different structures: upper and lower bainite. Upper bainite has a feathery structure. It is formed in the range of 500-350°C and consists of lath-shaped ferrite particles <1 µm thick and 5-10 µm wide, as well as thin cementite particles. The structure of upper bainite is characterized by higher hardness and strength, but lower ductility. Lower bainite has a needle-like martensite-like structure and is formed in the range of 350-200 °C. Lower bainite consists of fine particles of ε-carbides located in ferrite platelets. The bainite transformation never goes to completion. The structure always contains martensite and retained austenite. More preferable, in terms of performance characteristics, is the lower bainite structure. Products with such a structure are used in car construction, where parts experience shock-tensile stresses. The bainite hardening technology requires special hardening equipment. Additional materials on this technology can be found in the article “Technology of hardening for bainite.”
Cold treatment (5) is used for steels in which the temperature of the end of the martensitic transformation Mk is below room temperature.
High-speed steels, cemented parts, measuring instruments, and other particularly precise products are subjected to cold treatment. You can read more about this non-standard method of heat treatment in the article “Cold processing of steel parts”
To mainHARDENING
Hardening is used to give steel the highest hardness. Steel heated above the upper critical point will have the structure of austenite, in which carbon is in a dissolved state. With slow cooling below 723° C, austenite turns into pearlite, a eutectoid mixture of cementite and ferrite. In this structure, the cementite particles are relatively large. If carbon steel, which has a temperature above the upper critical point, is quickly cooled, i.e., hardened, then austenite will not have time to transform into pearlite and, having been supercooled to approximately 200 ° C, will transform into a new structure - martensite, which has a Rockwell hardness of Rc
= 60 - 64, while pearlite has a hardness of up to
Rc
- 24. Martensite resists abrasion well.
In simple carbon steels, when heated above 200°C, martensite begins to disintegrate and transform into the next structure - troostite. The martensite structure is unstable; when heated above 200° C, it tends to transform into a more stable structure - troostite-sorbitol and, finally, at temperatures above 720° C - into the most stable structure - pearlite. If it is necessary to preserve martensite in hardened carbon steel, then tempering should be done at a temperature not exceeding 200 - 220 ° C. It should be noted that martensite after tempering has better properties than untempered martensite, as a result of which the part becomes more elastic and less prone to warping during natural aging. In addition, tempered martensite has a slightly larger specific volume than quenched martensite. This is used in practice to repair worn ring-shaped tools. For example, if a ring-shaped part, hardened for martensite, has a hole size larger by 0.005-0.01 mm compared to the nominal size, then repeated tempering at 200-220 ° C (straw color tarnish) can reduce the hole diameter due to the transformation of hardened martensite into tempering martensite, increasing the volume of the part. The hardened martensite of carbon steels, in turn, has a larger specific volume compared to the original pearlite structure by approximately 1°/0. This means that the part after hardening for martensite increases in volume by almost 1% (for different carbon steels and different heat treatment methods, the increase in volume is different, but not higher than 1%). Therefore, ring-shaped parts made of carbon steel after hardening to martensite change dimensions: the diameter of the hole decreases, the total volume of the part increases. The increase in volume during hardening also affects the warping of the part and the occurrence of thermal stresses. During hardening, the surface layers of the part (corners, necks, recesses) cool first, and then the deeper layers of the metal. The deeper layers of the metal, cooling, fix the structure of martensite, the specific volume of which is greater than the specific volume of austenite and pearlite, but the increased volume has nowhere to expand, because the surface layers have already hardened and, in turn, created pressure (due to expansion) on the internal layers. As a result, large internal stresses are created in the metal, which not only cause warping of the part, but also often form cracks or completely tear the hardened part into several parts. These phenomena are observed especially when the original structure before hardening - pearlite was coarse-plate-like, and free cementite (in steel containing more than 0.8% carbon) was in the form of a network and these structures were not corrected before hardening. In alloy steels, unlike carbon steels, austenite, when cooling the steel from the quenching temperature, requires such rapid cooling to transform into martensite. And the more special impurities (tungsten, vanadium, nickel, chromium, etc.) contained in steel, the slower cooling this transition occurs. This explains that in special steel (with the same carbon content compared to carbon steel) when cooled in a weaker cooling medium than water (for example, mineral oil), the martensitic structure is fixed to a greater depth than in carbon steel when quenched in water. In carbon steel, the martensitic structure can be fixed to a depth of no more than 8 - 12 mm
, and in deeper layers subsequent structures have time to form - troostite, sorbite, and with a large cross-section, the initial structure before hardening - pearlite - is formed in the core, since the cooling rate of the core was sufficient to form a pearlite structure.
Thus, the slower transition of austenite to martensite in special steels creates conditions for a more uniform (in time) transition of structures both on the surface and in the deep layers of the part). The internal heat of the part does not allow the surface layers to cool quickly, and the rate of heat transfer from these layers to the cooling medium—the oil—is also not high. As a result, thermal stresses in special steel are several times lower than in carbon steel, and therefore, the warping of the part will be less. In addition, in special steels with a high content of special impurities (for example, high-speed steel grade P18), even when cooling in air, austenite does not have time to transform into martensite and 15–20% of it remains after complete cooling of the steel during quenching. But austenite has a specific volume smaller than the volume of the original structure before heating - ferlite, and due to this, the partial increase in the volume of the part during hardening from the formed martensite is reduced due to the remaining (residual) austenite. For hardening, special steels are heated to a higher temperature than carbon steels, since the transition to a solid solution—austenite—in special steels occurs over a wider temperature range. In addition, special steels are not as sensitive to overheating as carbon steels, and grain growth during prolonged exposure at the quenching temperature in alloy steels is several times slower than in carbon steel. If the hardening temperature of steel grades U10 and U12 is 760 - 770 ° C, then for alloy steels it ranges from 800 to 900 ° C, and for special steels of grades P18 and P9 it reaches 1250 - 1300 ° C. Decay of martensite after quenching during tempering in special steels also begin at higher temperatures than in carbon steels, and range from 300 to 700 ° C. In P18 steel, martensite begins to transform into troostite only at a temperature of 650 - 680 ° C, therefore this steel is tempered at a temperature of 550 ° C . High-speed steel is tempered not only to eliminate stresses, but also to increase hardness due to the transition of retained austenite to martensite. It was stated above that in high-speed steel, 15 - 20% of austenite does not have time to transform into martensite during quenching, as a result of which the hardness of the steel after quenching is Rc
= 56 - 60 and the durability of the cutting tool is low (the cutting edge will quickly become dull).
If such a tool is heated after hardening to 520 - 560 ° C and held at this temperature for 1 - 2 hours, then part of the retained austenite will transform into martensite. If you repeat this tempering 2-3 times, then each time part of the austenite will transform into martensite. As a result of repeated tempering, the amount of martensite will increase and the hardness of the tool will rise to Rc
= 61 - 64. Thus, we see that martensite in special steels is more stable than in carbon steels.
At the same time, it is stronger, more elastic and less fragile due to the presence of special impurities. As indicated, martensite at a certain temperature begins to transform into the troostite structure. Troostite is a transitional structure. The hardness of troostite is Rc
= 40 - 50, and the viscosity is higher than that of martensite.
If we want to obtain troostite during tempering by decomposition of martensite, then such a transition occurs in the temperature range 220 - 320 ° C for carbon steels and 300 - 700 ° C for special steels. If steel hardened to martensite is tempered at a higher temperature (for carbon, chromium and chromium-nickel steel 500 - 550 ° C), troostite will transform into the next structure - sorbitol. This structure is the best structure for parts subject to torsion and tension (engine connecting rod bolts, braces, etc.). The hardness of sorbitol is Rc
= 31 - 40. The resistance to torsion and rupture is significantly higher than in any other structure.
In a fracture, the part treated with sorbitol tempering has a pronounced fibrous structure. Hardening of large workpieces or parts (having a cross-section of more than 120 mm
) made of carbon steel practically does not improve the mechanical properties and is not economically profitable.
For such sections, special steels should be used, since they are highly hardenable and are more durable due to the presence of special impurities. Previous page
| table of contents | Next page |
Dependence of martensite hardness on carbon content
The hardness of steel after quenching depends on the hardness of martensite, which in turn depends on the carbon content. As the carbon content increases, the hardness after hardening of the steel also increases. The graphical dependence is shown in the figure.
Graph of martensite hardness versus carbon content
Solvent for alkyd enamel - https://www.dcpt.ru